Professional Documents
Culture Documents
Chemistry
Chemistry
Uploaded by
8n4g8nvhmmOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemistry
Chemistry
Uploaded by
8n4g8nvhmmCopyright:
Available Formats
03/11/23
rate
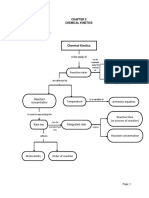
The rate of reaction is the amount of rectant converted to
product in a specified time period (per min per hour, per second) or the amount of product formed in a specified period of time
,
Some reactions that are important M an
to are either too fast or too slow
.
If a reaction is too fast it could be dangerous .
If a reaction is too slow ,
it may be not feasable or profitable .
,
The rate of reaction must either be increased or . Such
decreased reactions include many industrials and food manufacturing process that produce products that are important to use .
such products include feartilers , plastics , foods and medicine
The collision theory
In order for a reaction to take place the reactant particles must collide into each other
.
Every collision doesn't result in a reaction . Certain requirements must be met in order for
a reaction to take place
requirements
The collide
-
reactant particles must
- on collision the particles must have sufficent energy for reaction (activation energy)
-
reactant particles must be correctly oriented when collision occur
-
If collision is required for reaction to take place , the rate of reaction can increase by
-increasing the number of collisions of the reactant particles
~
increasing the number of reactant particles that have the activation energyt or the reaction
factors affecting Rate of reaction
The rate of reaction could be increased or decreased by the manipulation of certain conditions ·
These factors or conditions are
-
concentration
pressure
-
temperature
-sur face area
catalyst
clight)
concentration
You might also like
- Dokumen - Tips - Fanuc Robotics System R j3 Controller S 430i Series Mechanical Unit Maintenance Manual Marm3s43009801e Rev B PDFDocument4 pagesDokumen - Tips - Fanuc Robotics System R j3 Controller S 430i Series Mechanical Unit Maintenance Manual Marm3s43009801e Rev B PDFMabrouk GuezatiNo ratings yet
- Amc Offer 500 Kva & 380 Kva DGDocument8 pagesAmc Offer 500 Kva & 380 Kva DGabhibawa100% (3)
- Atlas Copco-Other Oil-Injected Rotary Screw Compressors en (Search-Manual-Online - Com)Document9 pagesAtlas Copco-Other Oil-Injected Rotary Screw Compressors en (Search-Manual-Online - Com)Anonymous vRyv4wM100% (1)
- t4 SC 1107 Aqa Gcse Chemistry Separate Science Unit 6 The Rate and Extent of Chemical Change KN Ver 7Document4 pagest4 SC 1107 Aqa Gcse Chemistry Separate Science Unit 6 The Rate and Extent of Chemical Change KN Ver 7Sarah KKCNo ratings yet
- OUTLINE - Reactions & Molecular CollisionsDocument2 pagesOUTLINE - Reactions & Molecular CollisionsJasmine Mary Issa QuezonNo ratings yet
- Factors Affecting Rate of Reaction Collision TheoryDocument1 pageFactors Affecting Rate of Reaction Collision TheoryMei XiangNo ratings yet
- Chapter 9Document18 pagesChapter 9JeromeNo ratings yet
- Chemical KineticsDocument7 pagesChemical Kinetics2100428No ratings yet
- Rate of Chemical Change KODocument3 pagesRate of Chemical Change KOAyesha RahmanNo ratings yet
- Chemistry - Kadar TindakbalasDocument40 pagesChemistry - Kadar Tindakbalasrashifah100% (1)
- Rate of Reaction - Freestate 2024 ComboDocument52 pagesRate of Reaction - Freestate 2024 ComboresegomohontiNo ratings yet
- Phainorc Reviewr DraftDocument5 pagesPhainorc Reviewr Draftejeraalaysa54No ratings yet
- c4 Rate of Reaction f5Document9 pagesc4 Rate of Reaction f5Rui Er LiewNo ratings yet
- Chemistry 3 Unit 2 ReviewerDocument6 pagesChemistry 3 Unit 2 ReviewerWEEA MAE CASTRONUEVONo ratings yet
- 8 - Reaction Kinetics IIDocument2 pages8 - Reaction Kinetics IINelsonNo ratings yet
- OMG 6 SainsTg 5 - Bab 04Document14 pagesOMG 6 SainsTg 5 - Bab 04AZIZAH BINTI EMBONG KPM-GuruNo ratings yet
- 1.5 KineticsDocument11 pages1.5 KineticsBhPO2023No ratings yet
- CHem InalsDocument3 pagesCHem InalsAbigail OconNo ratings yet
- Chemical KineticsDocument34 pagesChemical KineticsAditi KharatNo ratings yet
- Reactor Systems Improving Mass-Transfer-Limited Reactions PDFDocument6 pagesReactor Systems Improving Mass-Transfer-Limited Reactions PDFRajendraNo ratings yet
- Chemical Kinets PresentacionDocument32 pagesChemical Kinets PresentacionMarcos Javier Rojas FloresNo ratings yet
- Module 1 Kinetics of Materials Reading MaterialsDocument8 pagesModule 1 Kinetics of Materials Reading MaterialsmayNo ratings yet
- Chapter 6 - 2020 - 21 PDFDocument31 pagesChapter 6 - 2020 - 21 PDFAiman ZaidiNo ratings yet
- Bahria College Karachi Chapter # 8: Introduction To Chemical KineticsDocument15 pagesBahria College Karachi Chapter # 8: Introduction To Chemical KineticsNayan RoyNo ratings yet
- Ap ChemDocument6 pagesAp ChemholaholaholacarlottaNo ratings yet
- IB Chemistry - Unit 6 - Chemical Kinetics Study GuideDocument6 pagesIB Chemistry - Unit 6 - Chemical Kinetics Study GuideHamzah JoharNo ratings yet
- 228 - Pdfsam - DLP Textbook Chemistry Form 4Document34 pages228 - Pdfsam - DLP Textbook Chemistry Form 4MUHAMAD ASHRAF BIN NORDINNo ratings yet
- 2019 Yearly Plan Chemistry Form 5Document18 pages2019 Yearly Plan Chemistry Form 5Nor AqmarNo ratings yet
- Chemical KineticsDocument53 pagesChemical KineticsEuann MagtibayNo ratings yet
- Chemical KineticsDocument29 pagesChemical KineticsAditya PandeyNo ratings yet
- Chemical KineticsDocument40 pagesChemical KineticsHirdesh Sehgal100% (3)
- Rates of ReactionsDocument1 pageRates of Reactionsrda makeupNo ratings yet
- Collision Theory and Reaction RateDocument9 pagesCollision Theory and Reaction RateabdooufNo ratings yet
- Collision Theory and Reaction RateDocument9 pagesCollision Theory and Reaction RateKarissaNo ratings yet
- 95% ConversionDocument1 page95% ConversionLearn SomethingNo ratings yet
- Rates of ReactionDocument6 pagesRates of ReactionesotericgraphicapparelNo ratings yet
- Chemical Kinetics ReviewerDocument4 pagesChemical Kinetics ReviewervincentnagacNo ratings yet
- Kinetics PDFDocument5 pagesKinetics PDFAlexia LudlowNo ratings yet
- Rate of Reactions 18 April 2024Document46 pagesRate of Reactions 18 April 2024Amahle KudaNo ratings yet
- The Progress of Chemical Reactions: Lesson SummaryDocument3 pagesThe Progress of Chemical Reactions: Lesson SummaryIbrahim AlmalikNo ratings yet
- Nursyazmin - 205285 - Hakimi - 205433 - Lab Report 1 PDFDocument17 pagesNursyazmin - 205285 - Hakimi - 205433 - Lab Report 1 PDFMuhammad ArifNo ratings yet
- Gen Chem ReviewerDocument9 pagesGen Chem ReviewerMalayao, Philip Jude M.No ratings yet
- Ap Chem Unit 5 Review PacketDocument11 pagesAp Chem Unit 5 Review Packetapi-77411869No ratings yet
- 21 - Quantitative KineticsDocument1 page21 - Quantitative KineticsAbbas HaiderNo ratings yet
- Chemistry Form 5Document3 pagesChemistry Form 5alliey75% (8)
- Test 10 ChemistryDocument21 pagesTest 10 Chemistry030929No ratings yet
- Chemical KineticsDocument71 pagesChemical KineticsLoraine Andrei DeLeonNo ratings yet
- Chem f4 Chap7Document4 pagesChem f4 Chap7Alya QistinaNo ratings yet
- #6 - Revision Sheet KineticsDocument1 page#6 - Revision Sheet KineticsRovik Jeremiah BrotherBear RobertNo ratings yet
- CHEM12 - C1802 - SRVS (Correct)Document2 pagesCHEM12 - C1802 - SRVS (Correct)xr aimNo ratings yet
- Scheme of Work Chemistry Form 5Document31 pagesScheme of Work Chemistry Form 5Dilla IderesNo ratings yet
- Enzyme Worksheet Answer KeyDocument3 pagesEnzyme Worksheet Answer KeyEnessa Yurkin100% (1)
- Chemical Kinetics..Document26 pagesChemical Kinetics..Ayyan FerozNo ratings yet
- Chapter 3 Chemical KineticsDocument46 pagesChapter 3 Chemical KineticsaadarshceoNo ratings yet
- ANACHEM WEEK 3 TransDocument4 pagesANACHEM WEEK 3 TransAnn Frencis Louise PalaoNo ratings yet
- Enzyme KineticsDocument3 pagesEnzyme KineticsJay GaleNo ratings yet
- Introducción A La Cinética EnzimáticaDocument29 pagesIntroducción A La Cinética EnzimáticaPablo Alejandro Araujo GrandaNo ratings yet
- WastedDocument9 pagesWastedMark Francis DaculanNo ratings yet
- Rangkuman TRK (Deva Punya)Document4 pagesRangkuman TRK (Deva Punya)gamalielNo ratings yet
- Batch and Semi-batch Reactors: Practical Guides in Chemical EngineeringFrom EverandBatch and Semi-batch Reactors: Practical Guides in Chemical EngineeringNo ratings yet
- Drilling Bits: Dr. Gaurav Pandey Assistant Professor UpesDocument78 pagesDrilling Bits: Dr. Gaurav Pandey Assistant Professor UpeshassanNo ratings yet
- Zeta RevDocument46 pagesZeta Revsloba68100% (1)
- Electro Hydraulic Forming ProcessDocument60 pagesElectro Hydraulic Forming ProcessShawn HunterNo ratings yet
- Mass Balance CPC Blue CrudeDocument4 pagesMass Balance CPC Blue Crudedinkad29No ratings yet
- Lesson Plan 6 Electrical Installation and Maintenance I. ObjectivesDocument6 pagesLesson Plan 6 Electrical Installation and Maintenance I. Objectivescecille mañacapNo ratings yet
- Boilermaker Practice ExamDocument8 pagesBoilermaker Practice ExamSastoe DubeNo ratings yet
- 4.3 Enthalpy ChangesDocument14 pages4.3 Enthalpy ChangesKaihlaNo ratings yet
- Noble Corporation: Noble Analyst Day Singapore May 17-18, 2011Document27 pagesNoble Corporation: Noble Analyst Day Singapore May 17-18, 2011Andi ayuNo ratings yet
- PG 1200-350 Combo Parts ManualDocument54 pagesPG 1200-350 Combo Parts ManualAlfiya Anam100% (2)
- Chernobyl DisasterDocument65 pagesChernobyl DisasterStefan RaffsederNo ratings yet
- Increased Energy Yield of 50% at Flat Roof - Hanitsch Quaschning PDFDocument4 pagesIncreased Energy Yield of 50% at Flat Roof - Hanitsch Quaschning PDFshibly anastasNo ratings yet
- 07 Fluid CouplingDocument18 pages07 Fluid Couplingananttomar80361No ratings yet
- R6121e Mopn 0102Document14 pagesR6121e Mopn 0102zain shafiqNo ratings yet
- Karcher K 1.302 Manual 5961-0550Document24 pagesKarcher K 1.302 Manual 5961-0550vk_lozaNo ratings yet
- Baron Annual Inspection ChecklistDocument20 pagesBaron Annual Inspection Checklistalorena.leon21No ratings yet
- Booster in Sunscreen INOLEX PDFDocument18 pagesBooster in Sunscreen INOLEX PDFrenatoporangaNo ratings yet
- Serway PSE Quick ch32Document15 pagesSerway PSE Quick ch32Ràhuł MathiasNo ratings yet
- A7 Mindray Service Manual 12.0Document544 pagesA7 Mindray Service Manual 12.0Usama HassanNo ratings yet
- Thermodynamics 2 - Quiz #2 (Set A) : 1 1-k 1 K 2 1-k 2 KDocument2 pagesThermodynamics 2 - Quiz #2 (Set A) : 1 1-k 1 K 2 1-k 2 KCabagnot Piolo JuliusNo ratings yet
- Alak Kumar Guha: Curriculum VitaeDocument3 pagesAlak Kumar Guha: Curriculum VitaeKathak DancerNo ratings yet
- NDA Syllabus 2019 For Mathematics: Topic-Wise Distribution of Questions in MathsDocument4 pagesNDA Syllabus 2019 For Mathematics: Topic-Wise Distribution of Questions in Mathsrohit champNo ratings yet
- Chapter 1 Earth ScienceDocument5 pagesChapter 1 Earth ScienceLeizel MundoNo ratings yet
- Fluke 320 and 330 Series Clamp Meters: Technical DataDocument5 pagesFluke 320 and 330 Series Clamp Meters: Technical DataMilton NastNo ratings yet
- Spark Plug ReadingDocument7 pagesSpark Plug ReadingCostas GeorgatosNo ratings yet
- Grand Cherokee Error CodesDocument47 pagesGrand Cherokee Error Codeshossamezz18100% (3)
- Lab Report Djj20073 (Dad3b)Document11 pagesLab Report Djj20073 (Dad3b)khairul rizmanNo ratings yet
- Action JacDocument0 pagesAction Jacswoo323No ratings yet