Professional Documents
Culture Documents
Ideal Gas
Uploaded by
Sean GoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ideal Gas
Uploaded by
Sean GoCopyright:
Available Formats
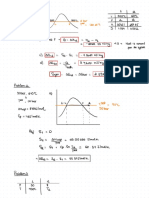
A 6 cubic meter tank contains helium at 400 K and is evacuated from
atmospheric pressure to a pressure of 740 mm Hg Vacuum. Determine
(a) The mass of helium remaining in the tank (b) the mass of helium
pumped out (c) The temperature of the remaining helium falls to 10 ◦C.
What is the pressure in kPa?
Ans. (a). m = 0.01925 kgm (b). 0.7123 kgm (c). P = 1.886 kPa
GIUEN:
12 18°
6m
:
V=
t 400K
= 1, 760mm
=
Hg
P2740mmAg-garge
=
REQ'D:
TANK
A. MHelium IN
B. MHELIUM PUMPED OUT
C. P2 IN KPG
S02'N:
SOLVE FOR m, (BEFORE)
mRT; m 1
PU=
=
RT
m=
elumt
RHELIM 2.0769
=
k
kym
-
-(101.325(kPa(t) (6(ms
-
(2.0769(2(400)K
K
kgm -
YUNG MASS BEFORE
m1 0.7317kgm-10
=
VACUUM.
MAEUACUATE SA
SOLVE FOR Me (AFTER) 760mmHg 29.92 inHg
=
14.7 PSi
=
=
PU=mRT; m DU
-
=
latm 101.325kPa
=
RT
pat
Patm-PUaC
and
Pabs =
:Pabs 760mmHg -740mmHg
=
20mm
-
Ag
Me =
- -
120 (mmHg (35)
(2.0769)(400) K
k
kym
-
M2 0.0193 kgm
=
SOLUE FOR M, (PUMPED OUTL
Mz m,
= -
M2
=
(0.7317-0.0193) kgm
My 0.7125kgm
=
SOLVE FOR T, @ 10°C
t
Puma W
kPa=
Is
v
273)k
m.(10 1.8906 1.8906kPa
+
=10.0193(kgm (2.0769),
=
=
-
(6) m3
You might also like
- 5 - Shafting ProblemsDocument3 pages5 - Shafting ProblemsIan ReyesNo ratings yet
- This Study Resource Was: Homework No.2 (MEE 340)Document7 pagesThis Study Resource Was: Homework No.2 (MEE 340)Kenneth SablayNo ratings yet
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Thermal Power PlantDocument34 pagesThermal Power PlantRama Krishna KariNo ratings yet
- Engineering-Fluid-Mechanics-11th-Edition SolutionsDocument136 pagesEngineering-Fluid-Mechanics-11th-Edition Solutionskevin shiNo ratings yet
- Filled Composite ColumnDocument1 pageFilled Composite ColumnAlphaNo ratings yet
- Preboard2 Psad Situation 2 Pile FootingDocument1 pagePreboard2 Psad Situation 2 Pile FootingAngelice Alliah De la CruzNo ratings yet
- Tank Venting According API 2000Document27 pagesTank Venting According API 2000Pouria Sabbagh100% (1)
- Oral Disease: Dental CariesDocument8 pagesOral Disease: Dental CariesAnna PruteanuNo ratings yet
- Fluid Mechanics (Unknown) SolnDocument136 pagesFluid Mechanics (Unknown) SolnErik BaldadoNo ratings yet
- Strema ReviewerDocument64 pagesStrema ReviewerRiri Agustin100% (1)
- CaissonDocument2 pagesCaissonjorge01No ratings yet
- Life of Celwenkosini by Nomfundo MbheleDocument792 pagesLife of Celwenkosini by Nomfundo Mbheleanelelerato70No ratings yet
- Solution Manual For Thermodynamics For Engineers 1st Edition by Kroos and PotterDocument7 pagesSolution Manual For Thermodynamics For Engineers 1st Edition by Kroos and PotterGetbook solutions 123No ratings yet
- Tutorial 5Document3 pagesTutorial 5NUR ATHIRAH NAJIHAH BINTI SAMEON / UPMNo ratings yet
- Qu=Cnc+Qnq+0.5 Γbnγ: Computation Of Ultimate Bearing Capacity: Terzhagi'S EquationDocument3 pagesQu=Cnc+Qnq+0.5 Γbnγ: Computation Of Ultimate Bearing Capacity: Terzhagi'S EquationJeremy Mark SorianoNo ratings yet
- Ib 1 - 14.223 - cm2 Dina Lbar 1x10 cm2: EXA F 10Document4 pagesIb 1 - 14.223 - cm2 Dina Lbar 1x10 cm2: EXA F 10Jose GalanNo ratings yet
- Design of Truss Malika Ali BSCE-5ADocument78 pagesDesign of Truss Malika Ali BSCE-5AUdat Hakeem Malang AmirNo ratings yet
- Airconditioning PSE SolutionDocument9 pagesAirconditioning PSE SolutionPablo BernadasNo ratings yet
- Thermo Lec 03Document2 pagesThermo Lec 03jgNo ratings yet
- Monteron Jaji C3Document18 pagesMonteron Jaji C3John Lloyd TulopNo ratings yet
- Perencanaan Kolom Bulat: Data: 2Document7 pagesPerencanaan Kolom Bulat: Data: 2Silviano Da Coneicicao AparicoNo ratings yet
- Week 11 AssessmentDocument9 pagesWeek 11 AssessmentVenrick CamposNo ratings yet
- Poder Calorífico: 25g (Plata)Document9 pagesPoder Calorífico: 25g (Plata)Marti GalanteNo ratings yet
- Concreto 3dwin REV 2004Document14 pagesConcreto 3dwin REV 2004EDWIN BALBUENANo ratings yet
- 3 PGH Pain: MMHG Gauge H Pbiood ( (Document1 page3 PGH Pain: MMHG Gauge H Pbiood ( (Rebeca GarzaNo ratings yet
- TablasDocument1 pageTablasMaria Del Pilar ChoqueNo ratings yet
- Tutorial 2 (207540)Document9 pagesTutorial 2 (207540)Wan Nur AtikahNo ratings yet
- C3 SolutionsDocument2 pagesC3 SolutionsCharlie SumagaysayNo ratings yet
- Pgiven: Given: HDocument1 pagePgiven: Given: HSean GoNo ratings yet
- Power Cycles IceDocument11 pagesPower Cycles Icecarlverano0428No ratings yet
- Probset Lab2Document2 pagesProbset Lab2maglasangpatrickryan123No ratings yet
- Serie 1 Parcial TermodinámicaDocument11 pagesSerie 1 Parcial TermodinámicaJimena GarcíaNo ratings yet
- TONGO - Final ProjectDocument71 pagesTONGO - Final ProjectJhon Nicko TongoNo ratings yet
- Basic Calculation of Heat Exchanger Shell and TubesDocument5 pagesBasic Calculation of Heat Exchanger Shell and TubesPutra EngineerNo ratings yet
- QL 30 KN/M QD 23 KN/M + Berat Sendiri D HDocument5 pagesQL 30 KN/M QD 23 KN/M + Berat Sendiri D HAdam FaisalNo ratings yet
- Design of Footing Dimension: Isolated Rectanguar Footing Irf-1 (L 1.5B)Document10 pagesDesign of Footing Dimension: Isolated Rectanguar Footing Irf-1 (L 1.5B)Amber ArellanoNo ratings yet
- Assignment 3 Solutions 2008Document6 pagesAssignment 3 Solutions 2008Matt GregorNo ratings yet
- Sual Kimi2Document66 pagesSual Kimi2Türk TürkNo ratings yet
- Gabarito Parcial - Lista 2Document3 pagesGabarito Parcial - Lista 2Paulo Sérgio Zanin JúniorNo ratings yet
- Tasca 5Document3 pagesTasca 5pj5t57cjvhNo ratings yet
- HW 12Document2 pagesHW 12haiNo ratings yet
- Fatiga - Esfuerzo Medio Cero 1Document8 pagesFatiga - Esfuerzo Medio Cero 1CHUGAR VILLCA CHRISTIAN GERMANNo ratings yet
- .Ee/O-Bm4G 2.B2E1O5Hx - Cklal0.332Reyhpr: IpsascpcdtldtDocument2 pages.Ee/O-Bm4G 2.B2E1O5Hx - Cklal0.332Reyhpr: Ipsascpcdtldtบรรณสรณ์ ขวัญนุ้ยNo ratings yet
- Exercise (Sedimentation) PDFDocument20 pagesExercise (Sedimentation) PDFDivyashini MohanNo ratings yet
- Cap Portante Cohesivo DrenadoDocument2 pagesCap Portante Cohesivo DrenadoRUBEN DARIO VILLCA QUIÑONESNo ratings yet
- Diseño Por FlexionDocument4 pagesDiseño Por FlexionrNo ratings yet
- Cbe 40B HW5Document5 pagesCbe 40B HW5Huỳnh Huy ĐắcNo ratings yet
- Partie Raper Signe: PlohoDocument9 pagesPartie Raper Signe: PlohoJeshNo ratings yet
- Parcial 1 TermodinámicaDocument4 pagesParcial 1 TermodinámicaGaspar SantiagpNo ratings yet
- PipeDocument3 pagesPipeCasper James SamsonNo ratings yet
- Thrust Formula PDFDocument5 pagesThrust Formula PDFchaktimewpNo ratings yet
- Desain JembatanDocument3 pagesDesain JembatandedyNo ratings yet
- Hwsoln 02Document7 pagesHwsoln 02exergy 33No ratings yet
- Design of 145kV SF6Document7 pagesDesign of 145kV SF6Newton AdhikariNo ratings yet
- CalculeDocument11 pagesCalculeradu2205No ratings yet
- Tutorial FastenerDocument14 pagesTutorial FastenerFaris DanialNo ratings yet
- AE1222-II Formula Sheet Aircraft Design Version 25-3-2021Document4 pagesAE1222-II Formula Sheet Aircraft Design Version 25-3-2021daniel dusNo ratings yet
- Práctica 4Document15 pagesPráctica 4Angel VolquezNo ratings yet
- MEMOERIA DE CALCULO HoAo h5mDocument3 pagesMEMOERIA DE CALCULO HoAo h5mRamiro RapshonNo ratings yet
- Determine The (A) Temperature at A State of P 0.5 Mpa and H 2890 KJ/KG and (B) Degree SuperheatDocument1 pageDetermine The (A) Temperature at A State of P 0.5 Mpa and H 2890 KJ/KG and (B) Degree SuperheatSean GoNo ratings yet
- Example Problem Solutions - Chapter 8Document18 pagesExample Problem Solutions - Chapter 8Nguyen Tien DungNo ratings yet
- Parts Catalog Cummins, 6LTAA8.9G2 - ESN 82312099 - CPL 3079-9Document1 pageParts Catalog Cummins, 6LTAA8.9G2 - ESN 82312099 - CPL 3079-9Hardiansyah SimarmataNo ratings yet
- Dialog AdmissionDocument3 pagesDialog AdmissionekaNo ratings yet
- 23 74 13 Air Handling UnitsDocument16 pages23 74 13 Air Handling UnitsSajidNo ratings yet
- PiousDocument37 pagesPiousHelplineNo ratings yet
- Lesson 3 The Ideal Gasq PDFDocument4 pagesLesson 3 The Ideal Gasq PDFireneNo ratings yet
- House of Prayer in PenangDocument6 pagesHouse of Prayer in PenangKLEBER RAMOSNo ratings yet
- Research Paper UCSPDocument7 pagesResearch Paper UCSPJohn Adrian PadriqueNo ratings yet
- Audit FundamentalsDocument37 pagesAudit Fundamentalsaqsa palijoNo ratings yet
- HeijunkaDocument7 pagesHeijunkaCyrano14No ratings yet
- Safe Work Practices On Low Voltage and Mechanical ApparatusDocument23 pagesSafe Work Practices On Low Voltage and Mechanical ApparatusUET MAINNo ratings yet
- Should The Age of Driving Be RaisedDocument2 pagesShould The Age of Driving Be RaisedhakimNo ratings yet
- Dissertation Report On: Marketing Strategy of Dabur Vatika Hair Oil & Dabur Chyawanprash'Document54 pagesDissertation Report On: Marketing Strategy of Dabur Vatika Hair Oil & Dabur Chyawanprash'su_pathriaNo ratings yet
- Report On Attendance: Magwawa Integrated SchoolDocument2 pagesReport On Attendance: Magwawa Integrated SchoolEeve YhoungNo ratings yet
- A Wild Night With My New Boss WattpadDocument80 pagesA Wild Night With My New Boss WattpadLost And WanderNo ratings yet
- An Induced Pluripotent Stem Cell Line GZHMCi004 A Derived Fro - 2021 - Stem CeDocument4 pagesAn Induced Pluripotent Stem Cell Line GZHMCi004 A Derived Fro - 2021 - Stem CeFrankenstein MelancholyNo ratings yet
- Ilovepdf Merged 2 PDFDocument307 pagesIlovepdf Merged 2 PDFAhmed ZidanNo ratings yet
- A Crazy Duck Cow My Dog and Cat Reading Comprehension For Grade 1Document4 pagesA Crazy Duck Cow My Dog and Cat Reading Comprehension For Grade 1Rika SuryaNo ratings yet
- Bna Swot Analysis ListDocument11 pagesBna Swot Analysis ListBenjie MartinezNo ratings yet
- CV - Fransiskus Xaverius Rawi - Engine CadetDocument2 pagesCV - Fransiskus Xaverius Rawi - Engine CadetYuda PebriansyahNo ratings yet
- Is Matter Around Us PureDocument25 pagesIs Matter Around Us PureGaurav MehndirattaNo ratings yet
- NCP ScribdDocument3 pagesNCP ScribdAngela AyalaNo ratings yet
- 3161 9711 1 SMDocument3 pages3161 9711 1 SMpuskesmas sidosermoNo ratings yet
- Industrial Visit TO Kothagudem Thermal Power PlantDocument23 pagesIndustrial Visit TO Kothagudem Thermal Power PlantAbhishek NarayanaNo ratings yet
- Post-Op Instructions For Immediate DenturesDocument1 pagePost-Op Instructions For Immediate DenturesMrunal DoiphodeNo ratings yet
- SimulationDocument2 pagesSimulationVin Mamuric Meneses100% (1)
- Farenhiet 451 - EssayDocument5 pagesFarenhiet 451 - Essayapi-275502795No ratings yet
- House Sitting AgreementDocument16 pagesHouse Sitting AgreementjamesNo ratings yet