Professional Documents
Culture Documents
Vapor Pressure Boiling Points
Vapor Pressure Boiling Points
Uploaded by
Канат Тютенов0 ratings0% found this document useful (0 votes)
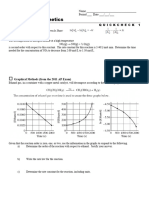
6 views1 pageThe document contains a graph showing the vapor pressure curves of two substances, A and B, as a function of temperature. It also contains 10 multiple choice questions that assess the reader's understanding of vapor pressure, normal boiling point, and the relationship between vapor pressure, temperature, and boiling point based on the graph.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document contains a graph showing the vapor pressure curves of two substances, A and B, as a function of temperature. It also contains 10 multiple choice questions that assess the reader's understanding of vapor pressure, normal boiling point, and the relationship between vapor pressure, temperature, and boiling point based on the graph.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views1 pageVapor Pressure Boiling Points
Vapor Pressure Boiling Points
Uploaded by
Канат ТютеновThe document contains a graph showing the vapor pressure curves of two substances, A and B, as a function of temperature. It also contains 10 multiple choice questions that assess the reader's understanding of vapor pressure, normal boiling point, and the relationship between vapor pressure, temperature, and boiling point based on the graph.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
Westminster H.S.
• AP Chemistry Name ________________________________
Period __ Date ___/___/___
10 Liquids, & Solids
V A P O R P R E S S U R E & B O I L I N G
The following graph shows vapor pressure curves for two substances, A and B:
1. What is the vapor pressure of A at 35C? 1.
2. What is the vapor pressure of B at 35C? 2.
3. At what temperature is the vapor pressure of A equal to 106.6 kPa? 3.
4. What is the vapor pressure of B at this temperature? 4.
5. At what temperature is the vapor pressure of B equal to 106.6 kPa? 5.
6. What is meant by “normal boiling point”? 6.
7. What is the normal boiling point of A? 7.
8. What is the normal boiling point of B? 8.
9. At what temperature would A boil if atmospheric pressure were 93.3 9.
kPa?
10. What would the atmospheric pressure have to be in order for B to boil at 10.
the temperature you gave as your answer to Question 9?
You might also like
- Https - Scholar - Vt.edu - Access - Content - Group - Practice Tests - Coming Soon - Practice Test 4 PDFDocument12 pagesHttps - Scholar - Vt.edu - Access - Content - Group - Practice Tests - Coming Soon - Practice Test 4 PDFEmmett GeorgeNo ratings yet
- Chapter-13 Assessment Gases Student EditableDocument5 pagesChapter-13 Assessment Gases Student EditableFabricio FernandezNo ratings yet
- Chem Preap Gas Laws Practice Test With AnswersDocument10 pagesChem Preap Gas Laws Practice Test With AnswersTony StarkNo ratings yet
- QuizDocument2 pagesQuizHelma Jabello AriolaNo ratings yet
- Q4 - 1ST Summative Test Science 10Document2 pagesQ4 - 1ST Summative Test Science 10Aj De CastroNo ratings yet
- Science Grade-10-ActivitiesDocument5 pagesScience Grade-10-ActivitiesDavie LegaspinaNo ratings yet
- A. P'V V'P C. VP V'P' B. VV' PP' DDocument2 pagesA. P'V V'P C. VP V'P' B. VV' PP' Dkikoy20No ratings yet
- II. Direction: Balance The Following Chemical EquationsDocument3 pagesII. Direction: Balance The Following Chemical EquationsHera S LinduganNo ratings yet
- II. Direction: Balance The Following Chemical EquationsDocument3 pagesII. Direction: Balance The Following Chemical EquationsHera S LinduganNo ratings yet
- 5-6 G10 ScienceDocument2 pages5-6 G10 Scienceisaacsamuel229No ratings yet
- Gas Laws Exam: Escribe Tu Nombre CompletoDocument4 pagesGas Laws Exam: Escribe Tu Nombre CompletoEver MendezNo ratings yet
- IS ChemistryDocument1 pageIS Chemistrycheese breadNo ratings yet
- Gas Laws Multiple Choice ReviewDocument3 pagesGas Laws Multiple Choice ReviewRolina Ruiz-LabaoNo ratings yet
- Notes Ws Phase Diagram Vapor Pressure KeyDocument4 pagesNotes Ws Phase Diagram Vapor Pressure KeyVanessa JabagatNo ratings yet
- Science 10Document3 pagesScience 10Anna Glodove SeredricaNo ratings yet
- Q4 Sci10 Assessment2-2Document2 pagesQ4 Sci10 Assessment2-2Jaezean Jules B. GomezNo ratings yet
- Instruction: Choose The Letter of The Best AnswerDocument3 pagesInstruction: Choose The Letter of The Best AnswerDaiseree SalvadorNo ratings yet
- Charle's LawDocument5 pagesCharle's Lawdark iceNo ratings yet
- Quiz 4.1 G10Document1 pageQuiz 4.1 G10BuhayParangLife BuhayParangLifeNo ratings yet
- Summative Test 4TH QDocument3 pagesSummative Test 4TH QFELIX ROBERT VALENZUELANo ratings yet
- ST1 Boyles Charles LawDocument1 pageST1 Boyles Charles LawnatashaeleanortamseNo ratings yet
- Quiz - Chapter 6Document5 pagesQuiz - Chapter 6dNo ratings yet
- Science 10 Q4 Module 1Document29 pagesScience 10 Q4 Module 1Maki Tuna100% (2)
- Pretest On Gas Laws: Multiple Choice: Choose The Letter of The Best AnswerDocument6 pagesPretest On Gas Laws: Multiple Choice: Choose The Letter of The Best AnswerClarence Mike BorjaNo ratings yet
- ASMEPPS Reviewer Chemistry 1Document2 pagesASMEPPS Reviewer Chemistry 1Morphetz ErtsNo ratings yet
- Formative To PrintDocument1 pageFormative To PrintshellaineNo ratings yet
- Chem Mid Exam Code 4Document6 pagesChem Mid Exam Code 4lenlucy13frNo ratings yet
- Vapor Pressure and Boiling Notes and WsDocument3 pagesVapor Pressure and Boiling Notes and Wskavleshgalhotra711No ratings yet
- Arya Nadgouda - Gases Review SheetDocument3 pagesArya Nadgouda - Gases Review SheetArya NadgoudaNo ratings yet
- Unit 7 Gas Laws Packet 2021Document40 pagesUnit 7 Gas Laws Packet 2021Alberto Laborte Jr.No ratings yet
- BOYLE'S LAW Group 1Document13 pagesBOYLE'S LAW Group 1Erich UntalanNo ratings yet
- 4q-Science Semi-Final ExamDocument3 pages4q-Science Semi-Final ExamRoselda Icaro - BacsalNo ratings yet
- Monthly Test GasesDocument3 pagesMonthly Test GasesTristan PereyNo ratings yet
- Summative and Perf Task Q4 Mod 2 1Document2 pagesSummative and Perf Task Q4 Mod 2 1Albert FragaNo ratings yet
- Final Exam in Science 10Document4 pagesFinal Exam in Science 10Daiseree SalvadorNo ratings yet
- 4th Quarter Test Science 10Document5 pages4th Quarter Test Science 10Precy CoNo ratings yet
- GC1 Mod Quiz 5.1 PDFDocument2 pagesGC1 Mod Quiz 5.1 PDFRewinEnvergaNo ratings yet
- Practice Exam III Chap5-6Document4 pagesPractice Exam III Chap5-6Jovenil BacatanNo ratings yet
- Test Ch.10: Multiple ChoiceDocument6 pagesTest Ch.10: Multiple ChoiceMj LeeNo ratings yet
- Unit Practice Test: Gas Laws: Multiple ChoiceDocument8 pagesUnit Practice Test: Gas Laws: Multiple Choiceanj pianoNo ratings yet
- LEARNING ACTIVITY SHEET 4 (Boyle's Law) - Science 10 (4th Quarter)Document2 pagesLEARNING ACTIVITY SHEET 4 (Boyle's Law) - Science 10 (4th Quarter)cherrymaeregalario2001No ratings yet
- Chemistry Quiz. Grade 10Document1 pageChemistry Quiz. Grade 10Ezekiel LapitanNo ratings yet
- General Chemistry 1: First Quarter-Module 9: Gas LawsDocument24 pagesGeneral Chemistry 1: First Quarter-Module 9: Gas LawsElysha Mae RamirezNo ratings yet
- JH EcampusUpload SubjectNote STUDY of GAS LAWSDocument2 pagesJH EcampusUpload SubjectNote STUDY of GAS LAWSdiamehta1410No ratings yet
- Heating CurveDocument1 pageHeating CurveКанат ТютеновNo ratings yet
- Multiple Choice: Choose The Letter of The Best Answer.: B. Inversely ProportionalDocument7 pagesMultiple Choice: Choose The Letter of The Best Answer.: B. Inversely ProportionalClarence Mike BorjaNo ratings yet
- Final QuestionnerDocument3 pagesFinal QuestionnerNica Mae MoralesNo ratings yet
- Post Test Gas LawDocument4 pagesPost Test Gas LawSmb RichieNo ratings yet
- Chem Preap Gas Laws Practice Test With AnswersDocument10 pagesChem Preap Gas Laws Practice Test With AnswersChristine GalsimNo ratings yet
- Fourth Grading Exam Boy Les LawDocument3 pagesFourth Grading Exam Boy Les Lawjerieljade.talabonNo ratings yet
- Fourth Grading Exam Boy Les LawDocument3 pagesFourth Grading Exam Boy Les Lawjerieljade.talabonNo ratings yet
- q4 Quiz 2Document2 pagesq4 Quiz 2Daniella CernaNo ratings yet
- Science 10 - Module Q4 SDO Malabon CityDocument29 pagesScience 10 - Module Q4 SDO Malabon CityHanna AngelaNo ratings yet
- Pre Test ScratchDocument4 pagesPre Test ScratchRAYMUND RODILLONo ratings yet
- TQ - Q4 - SCIENCE - 10 - Aster Madalla - Asterio MadallaDocument11 pagesTQ - Q4 - SCIENCE - 10 - Aster Madalla - Asterio MadallajoashcrixzNo ratings yet
- Navotas National High School: Is Known As P V P VDocument4 pagesNavotas National High School: Is Known As P V P Vmarco medurandaNo ratings yet
- Fourth Quarter Departmental TestDocument3 pagesFourth Quarter Departmental Testjoy grace jaraNo ratings yet
- Post Test 4th QuarterDocument2 pagesPost Test 4th QuarterRAYMUND RODILLONo ratings yet
- Science 10Document2 pagesScience 10Louie Jan SarnoNo ratings yet
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterFrom EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterRating: 5 out of 5 stars5/5 (1)
- Station Review AnswersDocument5 pagesStation Review AnswersКанат ТютеновNo ratings yet
- Le Chateliers Principle PracticeDocument1 pageLe Chateliers Principle PracticeКанат ТютеновNo ratings yet
- Quick Check 1 and 2Document2 pagesQuick Check 1 and 2Канат ТютеновNo ratings yet
- Practice Test W AnswersDocument3 pagesPractice Test W AnswersКанат ТютеновNo ratings yet
- Two Points AnswersDocument2 pagesTwo Points AnswersКанат ТютеновNo ratings yet
- Solution CompositionDocument2 pagesSolution CompositionКанат ТютеновNo ratings yet
- Mole and Equation PracticeDocument1 pageMole and Equation PracticeКанат ТютеновNo ratings yet
- Chapter 7 FULL PresentationDocument134 pagesChapter 7 FULL PresentationКанат ТютеновNo ratings yet
- IMF NotesDocument23 pagesIMF NotesКанат ТютеновNo ratings yet
- Quick Check 1Document1 pageQuick Check 1Канат ТютеновNo ratings yet
- Le Chateliers Principle Practice 2Document2 pagesLe Chateliers Principle Practice 2Канат ТютеновNo ratings yet
- Introduction To EnergyDocument2 pagesIntroduction To EnergyКанат ТютеновNo ratings yet
- IMF TrendsDocument1 pageIMF TrendsКанат ТютеновNo ratings yet
- IMF WorksheetDocument3 pagesIMF WorksheetКанат ТютеновNo ratings yet
- Heating CurveDocument1 pageHeating CurveКанат ТютеновNo ratings yet
- Hess S Law NotesDocument2 pagesHess S Law NotesКанат ТютеновNo ratings yet
- Handwarmer Lab Write-Up SampleDocument1 pageHandwarmer Lab Write-Up SampleКанат ТютеновNo ratings yet
- End Point CalculationsDocument2 pagesEnd Point CalculationsКанат ТютеновNo ratings yet
- Energies of Solution FormationDocument2 pagesEnergies of Solution FormationКанат ТютеновNo ratings yet
- AP PPT CH 11Document68 pagesAP PPT CH 11Канат ТютеновNo ratings yet
- End Point Calculations AnswersDocument2 pagesEnd Point Calculations AnswersКанат ТютеновNo ratings yet
- Chapter 13 Study QuestionsDocument2 pagesChapter 13 Study QuestionsКанат ТютеновNo ratings yet
- Ch14 PH CalculationsDocument1 pageCh14 PH CalculationsКанат ТютеновNo ratings yet
- Colligative Properties NotesDocument2 pagesColligative Properties NotesКанат ТютеновNo ratings yet
- Chapter 7 AP PresentationDocument57 pagesChapter 7 AP PresentationКанат ТютеновNo ratings yet
- AP Chem - Sections 1.2-1.3Document7 pagesAP Chem - Sections 1.2-1.3Канат ТютеновNo ratings yet
- AP PPT CH 9 AP OnlyDocument42 pagesAP PPT CH 9 AP OnlyКанат ТютеновNo ratings yet
- Chapter 7 Quick Check 3Document1 pageChapter 7 Quick Check 3Канат ТютеновNo ratings yet
- Buffer CalculationsDocument2 pagesBuffer CalculationsКанат ТютеновNo ratings yet
- Ch14 Quick Check 3Document1 pageCh14 Quick Check 3Канат ТютеновNo ratings yet