Professional Documents
Culture Documents
SHORT PROCEDURE Precipitation Titration
SHORT PROCEDURE Precipitation Titration
Uploaded by
anasriyas55Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
SHORT PROCEDURE Precipitation Titration
SHORT PROCEDURE Precipitation Titration
Uploaded by
anasriyas55Copyright:
Available Formats
SHORT PROCEDURE
EXPERIMENT : 06. Conductometric titration using BaCl2Vs. Na2SO4 using conductivity meter.
I. Aim: To….. write the question.
II. Procedure :
Types of Solution
Burette Solution Na2SO4
Beaker solution BaCl2 – 20 ml of the given solution and 20 ml of dis.water
Indicator No indicator
End Ponit Conductance decreases first and end point reaches increases sharply
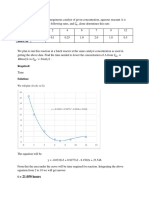
Scale X axis – Volume of Na2SO4 added (ml)
Y axis – Conductance (mho)
Materials :required MODEL GRAPH
1. Conductivity Meter Table : NaOH vs HCl
2. Conductivity cell
3. Beaker 100ml Volume of Conductance
4. Burette 50 ml Na2SO4 (mho)
5. Glass rod added(ml)
6. Measuring cylinder 50 ml
7. Distilled water 0
Calculation 3
Volume of Na2SO4 = --------ml [from graph]
Strength of Na2SO4 = 0.1 N 4
Volume of BaCl2taken = 20 ml 5
Strength of HCl = (-------x0.1) /20
6
∴ Strength of BaCl2 = ----------N
7
∴Total amount of BaCl2present in the given solution
8
= Strength of BaCl2 x Equivalent
9
weight of BaCl2
= -----------N x 122.14 10
= -----------gms. 11
12
13
Result
14
The total amount of BaCl2present in the given solution=--------- gms.
You might also like
- IS 1786 2008 - TMT BarDocument22 pagesIS 1786 2008 - TMT Barananda_beloshe75No ratings yet
- Erosion Modelling of The 120-MM M256/m829a2 Gun SystemDocument30 pagesErosion Modelling of The 120-MM M256/m829a2 Gun SystemOliver PásztorNo ratings yet
- Sistem M 11000 Alutherm PlusDocument159 pagesSistem M 11000 Alutherm PlusAlb AlbNo ratings yet
- Electrochemical Processes in Biological SystemsFrom EverandElectrochemical Processes in Biological SystemsAndrzej LewenstamNo ratings yet
- Objective: Determination of Partition Co-Efficient of Acetic Acid Between Water and N-ButanolDocument5 pagesObjective: Determination of Partition Co-Efficient of Acetic Acid Between Water and N-ButanolSUDIPA KONER100% (1)
- Analysis of Carbonate MixturesDocument6 pagesAnalysis of Carbonate MixturesKimNo ratings yet
- Kinetics (Gjjkkkgty)Document5 pagesKinetics (Gjjkkkgty)Chrysler Kane DepnagNo ratings yet
- University of Southern Philippines Foundation Salinas Drive, Lahug, Cebu CityDocument9 pagesUniversity of Southern Philippines Foundation Salinas Drive, Lahug, Cebu CityKate EvangelistaNo ratings yet
- Cape Chemistry Lab CompressDocument6 pagesCape Chemistry Lab CompressDesmond JonesNo ratings yet
- Chemistry RecordDocument22 pagesChemistry RecordprinceNo ratings yet
- Experiment 3 Conductometry HCLDocument5 pagesExperiment 3 Conductometry HCLMayank BajajNo ratings yet
- Chem Lab Manual - Partly Corrected PDFDocument29 pagesChem Lab Manual - Partly Corrected PDFFatin MahtabNo ratings yet
- Applied Chemistry Laboratory Manual: CY19143 Common To I Sem. B.E. - Eee & Cse and B.Tech.-ItDocument52 pagesApplied Chemistry Laboratory Manual: CY19143 Common To I Sem. B.E. - Eee & Cse and B.Tech.-ItAbcdNo ratings yet
- Chem Lab ManualDocument63 pagesChem Lab ManualBala NandaNo ratings yet
- Lab Report #4Document2 pagesLab Report #4Mad BasblaNo ratings yet
- A00830666 - Lab Report - BatchDocument13 pagesA00830666 - Lab Report - BatchJocelyn garcia gonzalezNo ratings yet
- Experiment 1: Determination of Na Co and Naoh in A Mixture by TitrationDocument8 pagesExperiment 1: Determination of Na Co and Naoh in A Mixture by TitrationSaurabh RajNo ratings yet
- Chem Exp 2Document2 pagesChem Exp 2Himani UppalNo ratings yet
- HCL Vs Na2CO3Document3 pagesHCL Vs Na2CO3mehul chakrabartiNo ratings yet
- Analyzing The Percentage of Acid in Vinegar: CHE 101L General Chemistry LabDocument7 pagesAnalyzing The Percentage of Acid in Vinegar: CHE 101L General Chemistry LabPinaki RanjanNo ratings yet
- B.Tech Chemistry LABORATORY (18CYB101J) - 2019Document28 pagesB.Tech Chemistry LABORATORY (18CYB101J) - 2019Saurabh Raj0% (1)
- Determination of Chloride by Volhard and Mohr MethodDocument6 pagesDetermination of Chloride by Volhard and Mohr MethodShane AmolarNo ratings yet
- Lab ManualDocument32 pagesLab ManualAyush GoyalNo ratings yet
- Expt 3 Partition Coefficient 1Document4 pagesExpt 3 Partition Coefficient 1Purnima NaskarNo ratings yet
- Cape Chemistry LabDocument6 pagesCape Chemistry LabArvin SayotoNo ratings yet
- Equivalent Weight Normality Strength in GM./LTDocument2 pagesEquivalent Weight Normality Strength in GM./LTvasu patelNo ratings yet
- 11th Experiment-3Document2 pages11th Experiment-3theinvisibleminecrafterNo ratings yet
- Cbse 11 Volumetric Analysis 1Document2 pagesCbse 11 Volumetric Analysis 1ShreyaNo ratings yet
- Final ExamS2009 PDFDocument7 pagesFinal ExamS2009 PDFAgatha BermudezNo ratings yet
- Chemistry 31 - Quantitative Analysis Exam #2, November 26, 2008Document6 pagesChemistry 31 - Quantitative Analysis Exam #2, November 26, 2008Agatha BermudezNo ratings yet
- Data and Results Exp-3Document1 pageData and Results Exp-3tuzzibooNo ratings yet
- Question Score A Chapter 1Document14 pagesQuestion Score A Chapter 1Dee -AdilaNo ratings yet
- ConductometeryDocument2 pagesConductometerysyedgaffarsyedrajjak1No ratings yet
- Chemistry 31 - Quantitative Analysis Exam #1, March 4, 2009Document4 pagesChemistry 31 - Quantitative Analysis Exam #1, March 4, 2009Agatha BermudezNo ratings yet
- Lab Manual Physical Pharmaceutics IDocument16 pagesLab Manual Physical Pharmaceutics IRubal ChahalNo ratings yet
- Example 1Document8 pagesExample 1jgolloberNo ratings yet
- 11th Experiment-5Document2 pages11th Experiment-5theinvisibleminecrafterNo ratings yet
- Chemistry 31 - Quantitative Analysis Final Exam, December 17, 2008Document6 pagesChemistry 31 - Quantitative Analysis Final Exam, December 17, 2008Agatha BermudezNo ratings yet
- Analysis of An Antacid Using Back Titration (CAPE LAB)Document5 pagesAnalysis of An Antacid Using Back Titration (CAPE LAB)AmeliaNo ratings yet
- Chemistry The Determination of An Unknow PDFDocument8 pagesChemistry The Determination of An Unknow PDFAbdullah Sabry AzzamNo ratings yet
- Practical Analytical 1 ,,chemistryDocument45 pagesPractical Analytical 1 ,,chemistryFadlin AdimNo ratings yet
- 2017 Regular LSDocument8 pages2017 Regular LSbraidihanadi19No ratings yet
- Isothermal Batch ReactorDocument5 pagesIsothermal Batch ReactorSrikanthNo ratings yet
- Final PBA Chemistry HSSC-I-1Document8 pagesFinal PBA Chemistry HSSC-I-121617No ratings yet
- Experiment No 1Document3 pagesExperiment No 1NEHA BANSALNo ratings yet
- Amount of Substance QPDocument29 pagesAmount of Substance QPduneloasherNo ratings yet
- Chem2exam2 PDFDocument6 pagesChem2exam2 PDFLouis ParrNo ratings yet
- CRE Experiments PDFDocument52 pagesCRE Experiments PDFBilal AhmadNo ratings yet
- LAb Report 4Document3 pagesLAb Report 4Faisal MumtazNo ratings yet
- Sample Experiment RecordDocument3 pagesSample Experiment RecordNemalnath reddy KasarapuNo ratings yet
- Physical Test - Answers - 05.07.2021Document4 pagesPhysical Test - Answers - 05.07.2021joydeep17590No ratings yet
- Lubna - Chemistry - 12 LSDocument4 pagesLubna - Chemistry - 12 LSkhattab994No ratings yet
- Batch ReactorDocument5 pagesBatch ReactorSayyeda Neha FatimaNo ratings yet
- Answers T-12 Test-10 (Set-C) XI Evening 01.11.2023Document2 pagesAnswers T-12 Test-10 (Set-C) XI Evening 01.11.2023Ojasva TabletNo ratings yet
- Titration Calculations AS Chem Prac 4 2024Document6 pagesTitration Calculations AS Chem Prac 4 2024nathanbushe1638No ratings yet
- Amjad Highschool Final 2021 GS-LSDocument3 pagesAmjad Highschool Final 2021 GS-LSMJ TarhiniNo ratings yet
- 521 Bench ChemicalDocument13 pages521 Bench ChemicalAnishah ChaudheryNo ratings yet
- 2.mass Transfer With - Without Chemical ReactionDocument7 pages2.mass Transfer With - Without Chemical ReactionAjeet KumarNo ratings yet
- KIM20E Homework.15.04.2021Document1 pageKIM20E Homework.15.04.2021Beyza SuvernNo ratings yet
- The Determination of A Solubility Product ConstantDocument6 pagesThe Determination of A Solubility Product Constantapi-551058017No ratings yet
- Expt09 2020A1PS0727PDocument2 pagesExpt09 2020A1PS0727PNAVYA GUPTANo ratings yet
- Requirement: K Chemical EquationDocument2 pagesRequirement: K Chemical EquationParth DhimanNo ratings yet
- Chemistry Coursework Self Heating CansDocument7 pagesChemistry Coursework Self Heating Canskqgcnxejd100% (2)
- Sas9 STM-005Document6 pagesSas9 STM-005mayasNo ratings yet
- AQA A Level Chem CH31 Practice Question AnswersDocument3 pagesAQA A Level Chem CH31 Practice Question AnswersMahebul MazidNo ratings yet
- Aalterpaint - Galva Wash - 02 - enDocument2 pagesAalterpaint - Galva Wash - 02 - enAnitha Grey'sNo ratings yet
- Topic 1 and 2-ChemicalKineticsDocument86 pagesTopic 1 and 2-ChemicalKineticsNOR AZAM BIN ENDOT / FSNo ratings yet
- Effects of Adulterated Palm Cooking Oil On The Quality of Fried Chicken NuggetsDocument11 pagesEffects of Adulterated Palm Cooking Oil On The Quality of Fried Chicken NuggetsKiruthick DonNo ratings yet
- 1 s2.0 S2468823123000317 MainDocument18 pages1 s2.0 S2468823123000317 Mainjsar1No ratings yet
- CHEM316 Report2Document13 pagesCHEM316 Report2Jake GerolagaNo ratings yet
- S500MCDocument2 pagesS500MCPankaj GuptaNo ratings yet
- 24 09 2022 - JR.C 120 - Jee Main - WTM 11 - Q.PaperDocument12 pages24 09 2022 - JR.C 120 - Jee Main - WTM 11 - Q.PaperMurari MarupuNo ratings yet
- Cach25C - Chemistry - Ii: Öpõkuqøn Dû Gßóõà Gßú?Document6 pagesCach25C - Chemistry - Ii: Öpõkuqøn Dû Gßóõà Gßú?Gopinathan MNo ratings yet
- Intext and Exercise Ch-1Document6 pagesIntext and Exercise Ch-1Anshu SinghNo ratings yet
- Environmental Lab ManualDocument30 pagesEnvironmental Lab ManualSaqibAliShahNo ratings yet
- Agastya Vol 1 Issue 1 2023Document17 pagesAgastya Vol 1 Issue 1 2023hermaticianNo ratings yet
- Effect of Cleaners and Sanitizers On Listeria Monocytogenes Attached To Product Contact SurfacesDocument6 pagesEffect of Cleaners and Sanitizers On Listeria Monocytogenes Attached To Product Contact SurfacesKhela Pagol ManushNo ratings yet
- Methods of Sampling and Test (Physical and Chemical) For Water and WastewaterDocument10 pagesMethods of Sampling and Test (Physical and Chemical) For Water and Wastewaterlabn6446No ratings yet
- 4 - Boyana AngelovaDocument14 pages4 - Boyana AngelovaLuis Ricardo Soto JimenezNo ratings yet
- Scientific Committee On Consumer Safety SCCS: Opinion On P-AminophenolDocument57 pagesScientific Committee On Consumer Safety SCCS: Opinion On P-AminophenolSHERLY KIMBERLY RAMOS JESUSNo ratings yet
- Electrochemical Plating of Cu-Sn Alloy in Non-Cyanide Solution To Substitute For Ni Undercoating LayerDocument31 pagesElectrochemical Plating of Cu-Sn Alloy in Non-Cyanide Solution To Substitute For Ni Undercoating LayeronlynameNo ratings yet
- Determination of Paracetamol in Formulations by Direct UV-SpectrophotometryDocument16 pagesDetermination of Paracetamol in Formulations by Direct UV-Spectrophotometry0087 นันทิชาNo ratings yet
- Prepration of InkDocument62 pagesPrepration of Inkaditya bunkarNo ratings yet
- 12 Beaker Glass Pyrex 100mlDocument1 page12 Beaker Glass Pyrex 100mlzulfadli zainalNo ratings yet
- I. Objectives: Write The LC Code For EachDocument6 pagesI. Objectives: Write The LC Code For EachMitzie BocayongNo ratings yet
- Ans & Sol - Chemistry (Class XII) - 28!02!2023Document20 pagesAns & Sol - Chemistry (Class XII) - 28!02!2023sharanakash06No ratings yet
- Scale Inhibition and Removal in Continuous Pulp Digesters: Sujit Banerjee and Tuan LeDocument4 pagesScale Inhibition and Removal in Continuous Pulp Digesters: Sujit Banerjee and Tuan LetiaNo ratings yet
- Arihant Coordination CompoundDocument3 pagesArihant Coordination CompoundUpasana NathNo ratings yet