Professional Documents
Culture Documents
P-Block DTS-6
P-Block DTS-6
Uploaded by
Rudra guptaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
P-Block DTS-6
P-Block DTS-6
Uploaded by
Rudra guptaCopyright:
Available Formats
Date Planned : __ / __ / __ Daily Tutorial Sheet-6 Expected Duration : 30 Min
Actual Date of Attempt : __ / __ / __ Level-2 Exact Duration :_________
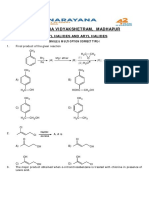
76. In the following reaction :
B(OH)3 H2O [B(OH)4 ] H
(A) B(OH)3 is a Lewis acid (B) B(OH)3 is amphoteric
(C) B(OH)3 is a Lewis base (D) None is correct
77. In the following statements, select the correct statement(s) :
(A) N(CH3 )3 has pyramidal structure (B) N(SiH3 )3 shows planar arrangement
(C) Both are correct (D) None is correct
78. The dipole moments of the given molecules are such that :
(A) BF3 NF3 NH3 (B) NF3 BF3 NH3
(C) NH3 NF3 BF3 (D) NH3 BF3 NF3
79. Boron carbide is used :
(A) In nuclear reactor to absorb neutrons (B) As an abrasive
(C) Both are correct (D) None is correct
80. Aqueous solution of borax reacts with two moles of acid. This is because of :
(A) formation of 2 mol of B(OH)3 only
(B) formation of 2 mol of [B(OH)4 ] only
(C) formation of 1 mol each of B(OH)3 and [B(OH)4 ]
(D) formation of 2 mol of [B(OH)4 ]
81. While testing BO33 , there is green-edged flame on heating the salt with conc. H2SO4 and CH3OH.
Green colour is of :
(A) (CH3 )3 B (B) (CH3O)3 B (C) B2O3 (D) H3BO3
82. The gaseous product(s) expected at room temperature by reaction of sodium boronhydride and boron
trifluoride under anhydrous condition is (are) :
(A) H2 (B) B2H3 and H2 (C) B2H6 (D) BH2F and H2
83. Choose the correct sequence for the geometry of the given molecules
Inorganic graphite, Borazole, B3O63 (NP : Non planar, P : Planar)
(A) NP, NP, NP (B) P, P, NP (C) NP, NP, P (D) NP, P, P
84. Borax is used as a cleansing agent because on dissolving in water, it gives :
(A) Alkaline solution (B) Acidic solution
(C) Bleaching solution (D) Basic solution
85. From B2H6 , all the following can be prepared except :
(A) B2 O 3 (B) H3BO3 (C) B2 (CH3 )6 (D) NaBH4
VMC | Level-2 136 DTS-6 | p-Block Elements-1
You might also like
- Educational Research Quantitative Qualitative and Mixed Approaches 6th Edition Johnson Solutions ManualDocument35 pagesEducational Research Quantitative Qualitative and Mixed Approaches 6th Edition Johnson Solutions Manualstrunttriose1xjj100% (21)
- Chapter 5 - Marketing Product, Services and BrandDocument84 pagesChapter 5 - Marketing Product, Services and BrandEida HidayahNo ratings yet
- PH-Electric-Rope-Shovel-Components (Splited)Document3 pagesPH-Electric-Rope-Shovel-Components (Splited)Oscar JimenezNo ratings yet
- This Study Resource Was: MAT 243 Project Three Summary ReportDocument8 pagesThis Study Resource Was: MAT 243 Project Three Summary ReportnellyNo ratings yet
- Gospel Centered DiscipleshipDocument3 pagesGospel Centered DiscipleshipSaraNo ratings yet
- P-Block DTS-4Document2 pagesP-Block DTS-4Rudra guptaNo ratings yet
- Chem QP PH-V Pet-3 Jee-M DT 11042024 - Test Anal - 240412 - 142616Document20 pagesChem QP PH-V Pet-3 Jee-M DT 11042024 - Test Anal - 240412 - 142616nagavali200006No ratings yet
- Organic Chemistry Guided Revision Plan-Score AdvancedDocument4 pagesOrganic Chemistry Guided Revision Plan-Score AdvancedNamchrahsiNo ratings yet
- Alcohols IIT PACEDocument19 pagesAlcohols IIT PACEDushyant GuptaNo ratings yet
- Exe 3Document29 pagesExe 3AkashGauravNo ratings yet
- Time: 1 Hrs Max. Marks: 98 Single Correct: 3 2 4 HG ZNDocument6 pagesTime: 1 Hrs Max. Marks: 98 Single Correct: 3 2 4 HG ZNlakshmi.vedanarayanan7785No ratings yet
- Alkyl Halides and Aryl HalidesDocument16 pagesAlkyl Halides and Aryl Halidesvardesh100% (1)
- FIITJEE Kukatpally Jee Mains Test PDFDocument16 pagesFIITJEE Kukatpally Jee Mains Test PDFmadhavNo ratings yet
- P-Block Elements-II - DTS 1Document2 pagesP-Block Elements-II - DTS 1Rudra guptaNo ratings yet
- Alkanes - Alkenes - Alkynes - DPP 3Document3 pagesAlkanes - Alkenes - Alkynes - DPP 3Vishal_93100% (1)
- (02-12-14) AlkenesDocument4 pages(02-12-14) Alkenessasi.curieNo ratings yet
- Sample Paper: Time: 90 Minutes Max. Marks: 35Document9 pagesSample Paper: Time: 90 Minutes Max. Marks: 35Harsh PatelNo ratings yet
- Nsec National Standard Examination in Chemistry: Class: Xi DATE: 22.11.2020Document13 pagesNsec National Standard Examination in Chemistry: Class: Xi DATE: 22.11.2020KritikaNo ratings yet
- Sheet 1Document11 pagesSheet 1Zooper lNo ratings yet
- D Block Compounds12thDocument7 pagesD Block Compounds12thRaju SinghNo ratings yet
- Quiz-Hydrocarbons-Snd SNDDocument6 pagesQuiz-Hydrocarbons-Snd SNDayesha sheikhNo ratings yet
- Boron Familiy MKADocument3 pagesBoron Familiy MKAyayNo ratings yet
- Wa0011.Document4 pagesWa0011.michaeldcosta414No ratings yet
- Jumbo Home Test-1 - FinalDocument59 pagesJumbo Home Test-1 - FinalSayali SachinNo ratings yet
- Quiz Organic 1Document6 pagesQuiz Organic 1ronakgupta332005No ratings yet
- Hydrocarbon Level:I KVPYDocument2 pagesHydrocarbon Level:I KVPYRAGHUL MNo ratings yet
- Exercise 2Document23 pagesExercise 2Tushar RajNo ratings yet
- IsomerismDocument21 pagesIsomerismkaransharma690No ratings yet
- BB YASMt DVEvy ZZi PR NYRDocument8 pagesBB YASMt DVEvy ZZi PR NYRarindamNo ratings yet
- Time: 1 Hrs Max. Marks: 87 Single Correct: OH OH OH OHDocument5 pagesTime: 1 Hrs Max. Marks: 87 Single Correct: OH OH OH OHlakshmi.vedanarayanan7785No ratings yet
- Exercise-01 Check Your Grasp: O CH HO HODocument29 pagesExercise-01 Check Your Grasp: O CH HO HOHet PrajapatiNo ratings yet
- Cbse Class 12Document15 pagesCbse Class 12ArchitaNo ratings yet
- Alcohols & EtherDocument18 pagesAlcohols & EtherRaju SinghNo ratings yet
- Part-I Chemistry Section-I: IIT JEE-2010 Paper-1 (Chemistry)Document19 pagesPart-I Chemistry Section-I: IIT JEE-2010 Paper-1 (Chemistry)SnehilNo ratings yet
- Carboxylic Acids and It's Derivative Aliphatic AminesDocument32 pagesCarboxylic Acids and It's Derivative Aliphatic AminesRaju SinghNo ratings yet
- Carbonyl Compounds 13thDocument21 pagesCarbonyl Compounds 13thRaju SinghNo ratings yet
- Acid & Amine-QuestionDocument6 pagesAcid & Amine-QuestionAnurag RamachandranNo ratings yet
- Chemistry Test PaperDocument12 pagesChemistry Test Papersougata_rintu9598No ratings yet
- I Am Sharing 'Assignment-3 Organic' With YouDocument28 pagesI Am Sharing 'Assignment-3 Organic' With YouKriti GargNo ratings yet
- Exercise-01 Check Your Grasp: O CH HO HODocument7 pagesExercise-01 Check Your Grasp: O CH HO HOChesta MalhotraNo ratings yet
- Time: 1 Hrs Max. Marks: 79 Single Correct: O Ph-C-ODocument6 pagesTime: 1 Hrs Max. Marks: 79 Single Correct: O Ph-C-Olakshmi.vedanarayanan7785No ratings yet
- GoodgDocument19 pagesGoodgTusharNo ratings yet
- Principle Related To Practical ChemistryDocument11 pagesPrinciple Related To Practical ChemistryEzhil MukilNo ratings yet
- Structure Identification & POCDocument8 pagesStructure Identification & POCHarshil rawal100% (1)
- Carboxylic Acid CPPDocument24 pagesCarboxylic Acid CPPGulshan kumarNo ratings yet
- Or-Carboxylic Acids and It's Derivative Aliphatic Amines 13th WADocument23 pagesOr-Carboxylic Acids and It's Derivative Aliphatic Amines 13th WAUppu EshwarNo ratings yet
- ChemistryDocument3 pagesChemistryDeena chemistNo ratings yet
- Test Organic Chemistry (25!11!2023)Document17 pagesTest Organic Chemistry (25!11!2023)kagini3173No ratings yet
- Carbonyl CompoundsDocument34 pagesCarbonyl CompoundsprinceNo ratings yet
- AHC TestDocument4 pagesAHC TestArpan KumarNo ratings yet
- Hydrocarbon-04 Solved ProblemsDocument14 pagesHydrocarbon-04 Solved ProblemsRaju SinghNo ratings yet
- Research PaperDocument16 pagesResearch PaperShounak DuttaNo ratings yet
- Goc & Eas Test-IiDocument7 pagesGoc & Eas Test-IiAniket GuptaNo ratings yet
- New Chaptest - Hydrocarbons For KKTYR02A01, KKTYW02F01 BatchDocument23 pagesNew Chaptest - Hydrocarbons For KKTYR02A01, KKTYW02F01 Batchiamxxxofficial86No ratings yet
- Carbohydrates and Amino AcidsDocument21 pagesCarbohydrates and Amino AcidsRamNo ratings yet
- 3 - Aldehydes and Ketones (Assignment) Booklet-2Document15 pages3 - Aldehydes and Ketones (Assignment) Booklet-2kraken monsterNo ratings yet
- NEET Haloalkanes and Haloarenes Important QuestionsDocument24 pagesNEET Haloalkanes and Haloarenes Important QuestionsDr. Shreya RoushanNo ratings yet
- Aldehydes and KetonesDocument29 pagesAldehydes and KetonesJiya singhNo ratings yet
- Organic QuestionsDocument51 pagesOrganic Questionshemab30851No ratings yet
- Chirality in Supramolecular Assemblies: Causes and ConsequencesFrom EverandChirality in Supramolecular Assemblies: Causes and ConsequencesF. Richard KeeneNo ratings yet
- P-Block Elements-II - DTS 2 Main (Archive) SolDocument2 pagesP-Block Elements-II - DTS 2 Main (Archive) SolRudra guptaNo ratings yet
- P-Block Elements-II - DTS 2 Main (Archive)Document2 pagesP-Block Elements-II - DTS 2 Main (Archive)Rudra guptaNo ratings yet
- Coordination Compounds - DTS 0 SolDocument10 pagesCoordination Compounds - DTS 0 SolRudra guptaNo ratings yet
- Coordination Compounds - DTS 0Document2 pagesCoordination Compounds - DTS 0Rudra guptaNo ratings yet
- Coordination Compounds - DTS 1Document2 pagesCoordination Compounds - DTS 1Rudra guptaNo ratings yet
- Periodic Properties Solution - DTS-1Document1 pagePeriodic Properties Solution - DTS-1Rudra guptaNo ratings yet
- Periodic Properties Solution - DTS-1 - JEE Main ArchiveDocument1 pagePeriodic Properties Solution - DTS-1 - JEE Main ArchiveRudra guptaNo ratings yet
- Periodic Properties Solution - DTS-2 - JEE Adv ArchiveDocument2 pagesPeriodic Properties Solution - DTS-2 - JEE Adv ArchiveRudra guptaNo ratings yet
- P-Block DTS-5Document2 pagesP-Block DTS-5Rudra guptaNo ratings yet
- 1111binomial Theorem DTS-5Document2 pages1111binomial Theorem DTS-5Rudra guptaNo ratings yet
- Binomial Theorem DTS-3111Document2 pagesBinomial Theorem DTS-3111Rudra guptaNo ratings yet
- P-Block DTS-3Document2 pagesP-Block DTS-3Rudra guptaNo ratings yet
- Binomial Theorem DTS-1111Document2 pagesBinomial Theorem DTS-1111Rudra guptaNo ratings yet
- Binomial Theorem DTS-41111Document2 pagesBinomial Theorem DTS-41111Rudra guptaNo ratings yet
- 3cce171c-d4d7-49b0-8dda-425b225fb1f5 (1)Document2 pages3cce171c-d4d7-49b0-8dda-425b225fb1f5 (1)j4606074No ratings yet
- Civilizations - Chapter 20-Africa&African Slave TradeDocument16 pagesCivilizations - Chapter 20-Africa&African Slave TradeSpencer KimNo ratings yet
- Operational Factors Human Performance - Attachment 1 - Flight Crew Interview Transcripts and Statements - Redacted-RelDocument262 pagesOperational Factors Human Performance - Attachment 1 - Flight Crew Interview Transcripts and Statements - Redacted-RelGF100% (1)
- Social End Ethical ProjectDocument20 pagesSocial End Ethical ProjectPrathmesh VashisthNo ratings yet
- Economics 11th Edition by Slavin ISBN Test BankDocument27 pagesEconomics 11th Edition by Slavin ISBN Test Bankamber100% (26)
- Saes S 020Document28 pagesSaes S 020dissanayake90kanishkaNo ratings yet
- 19 01 PDFDocument4 pages19 01 PDFAde JuandaNo ratings yet
- Aluminium - Handling, Storage, Maintenance and CleaningDocument7 pagesAluminium - Handling, Storage, Maintenance and CleaningKiran KarthikNo ratings yet
- Gpa & Cgpa CalculatorDocument7 pagesGpa & Cgpa CalculatorQaisKhanNo ratings yet
- Environmental Assessment, Old York RoadDocument208 pagesEnvironmental Assessment, Old York RoadThe Elmhurst TitanNo ratings yet
- Poles and Zeros: - The Dynamic Behavior of A Transfer Function Model Can Be Characterized by The Numerical Value of Its Poles and ZerosDocument43 pagesPoles and Zeros: - The Dynamic Behavior of A Transfer Function Model Can Be Characterized by The Numerical Value of Its Poles and ZerosSergio ReyesNo ratings yet
- 5 Wholesale Bluetooth & Portable Speakers For Your Sound-Obsessed CustomersDocument3 pages5 Wholesale Bluetooth & Portable Speakers For Your Sound-Obsessed CustomersMyrnaJoyPajoJaposNo ratings yet
- Urn Uvci 01 Ro O0el5r27d48j5xpyv19nx3vwpgq96k#jDocument2 pagesUrn Uvci 01 Ro O0el5r27d48j5xpyv19nx3vwpgq96k#jZAHARIA ANDREINo ratings yet
- BusinessDocument11 pagesBusinessCaba ArturoNo ratings yet
- Meeting Held On The 15-10-2021Document4 pagesMeeting Held On The 15-10-2021musa ballah koromaNo ratings yet
- Annual Performances of Reversible Air-To-Water Heat Pumps in Small PDFDocument11 pagesAnnual Performances of Reversible Air-To-Water Heat Pumps in Small PDFgustavoNo ratings yet
- Seven Segment Display InterfacingDocument3 pagesSeven Segment Display InterfacingKunal Sonone0% (1)
- Gold Exp C1 U1 Skills Test BDocument3 pagesGold Exp C1 U1 Skills Test BAyşe KarabekirNo ratings yet
- Effect of Vine Cutting Length and Angle-1035Document8 pagesEffect of Vine Cutting Length and Angle-1035Lindsay MyersNo ratings yet
- Joan Scott French Feminists and Rights of ManDocument22 pagesJoan Scott French Feminists and Rights of ManRaman KapoorNo ratings yet
- Mladendolar 2009Document12 pagesMladendolar 2009marxengelsNo ratings yet
- To Order Solution of This Paper. Whatsapp No. 9350849407 or Call No. 9312235086Document2 pagesTo Order Solution of This Paper. Whatsapp No. 9350849407 or Call No. 9312235086Santosh dhabekarNo ratings yet
- TD of EdtDocument28 pagesTD of EdtOussama SouidNo ratings yet
- W1 CementDocument2 pagesW1 CementLee LeeNo ratings yet