Professional Documents
Culture Documents
Covalentteacherv1 210875
Covalentteacherv1 210875
Uploaded by
Bank Yossy WoluslaweOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Covalentteacherv1 210875
Covalentteacherv1 210875
Uploaded by
Bank Yossy WoluslaweCopyright:
Available Formats
Review my learning 14–16 years
Available from rsc.li/3ITqz5E
Covalent bonding: teacher guidance
This resource forms part of the Review my learning series from the Royal Society of Chemistry.
Additional support for addressing misconceptions identified using these worksheets can be found at
rsc.li/3mm0IeW.
This worksheet assesses content from the 14–16 specifications. The content is a subset of the Bonding
worksheet and can be used to provide extra support for learners on covalent bonding. It can identify
learners’ knowledge gaps and misconceptions following the completion of that part of the curriculum.
The Covalent bonding worksheet covers the following topics:
interpreting diagrams representing covalent bonds
sharing electrons in covalent bonds
types of elements involved in covalent bonds.
If learners successfully answer questions on these topics, they can attempt the extension question
where they can complete a diagram representing the formation of a covalent bond.
There is only one level of this worksheet. Level 1 ( ) is a scaffolded worksheet in which learners
select words from a word list to complete sentences.
The worksheet can be used in a variety of ways:
as an assessment of learners’ knowledge at the beginning or end of a period of teaching
as an assessment of knowledge during a period of teaching and after learners have completed
the relevant section of the specification
as a revision tool prior to the relevant examination
as a refresher exercise for teachers or non-subject specialists.
There is also scope to use this worksheet to support learners who struggle with this type of bonding
particularly. These learners could then be encouraged to attempt the partially scaffolded Bonding
worksheets to reinforce their understanding.
The ‘What do I understand?’ page can be used both to identify areas needing whole class attention
and as an indicator for learners to help guide their revision.
The Teacher guidance provides model answers and guidance on learners’ misconceptions. Learners
can use the model answers to self- or peer assess.
© 2023 Royal Society of Chemistry
Review my learning 14–16 years
Available from rsc.li/3ITqz5E
Answers
Covalent bonding: knowledge check
1.1 covalent bonding
1.2 Covalent bonding – this bonding occurs between non-metal atoms. In a single covalent bond, a
pair of electrons is shared between two atoms. These shared electrons are found in the outer
shells of the atoms. Each atom contributes one electron to the shared pair of electrons.
Guidance: Note that in a dative (or coordinate) covalent bond, one of the atoms donates both
shared electrons.
Covalent bonding: test myself
2.1 non-metals only
2.2 Covalent bonds are strong.
2.3 The covalent molecule is carbon dioxide.
2.4 There are two electrons in a single covalent bond.
Guidance: It is a common misconception that covalent bonds are weak, but this is not the case.
Diamond, the hardest known substance, has covalent bonds only. Another misconception is that
atoms share one electron in a covalent bond. Learners need to understand that each atom
contributes one electron to a single covalent bond, so there are two electrons altogether.
Covalent bonding: feeling confident?
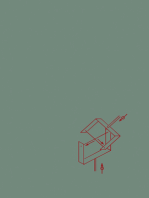
3.1
Guidance: The common misconceptions that learners have when drawing diagrams to represent
covalent bonds are only showing one electron being shared and adding extra electrons for
sharing. Encourage them to count the number of electrons in the outer shells to check they do
not exceed the permitted number for that shell, ensuring that the shared electrons are counted
for both atoms.
© 2023 Royal Society of Chemistry
Review my learning 14–16 years
Available from rsc.li/3ITqz5E
Covalent bonding: what do I understand?
Mini-topic Assessed via:
I can interpret diagrams representing covalent bonds. Q1.1
I know that electrons are shared in covalent bonds. Q1.2, Q1.2, Q2,4, Q3.1
I know the types of elements involved in covalent bonds. Q1.2, Q2.1, Q2.3
Feeling confident? topics Assessed via:
I can complete a diagram to represent the formation of a covalent bond Q3.1
© 2023 Royal Society of Chemistry
You might also like
- Lesson Plan For LEWIS DOT STRUCTUREDocument6 pagesLesson Plan For LEWIS DOT STRUCTUREHAROLD PAYUNAN100% (2)
- Bonding Unit PlanDocument64 pagesBonding Unit Planapi-366989204No ratings yet
- Flipped Lesson Plan Chemical BondingDocument4 pagesFlipped Lesson Plan Chemical Bondingapi-253202696100% (2)
- Atoms and Molecules WorksheetDocument1 pageAtoms and Molecules WorksheetBank Yossy Woluslawe60% (5)
- Simulation BondingDocument12 pagesSimulation BondingCristina Nicomedes Aguinaldo0% (2)
- Physical Science: Quarter 3 - Module 2: Molecular PolarityDocument16 pagesPhysical Science: Quarter 3 - Module 2: Molecular PolarityJuanits Bugay100% (9)
- Metallicteacherv1 385939Document4 pagesMetallicteacherv1 385939Bank Yossy WoluslaweNo ratings yet
- Adaptive Teaching Guide: Private Education Assistance CommitteeDocument6 pagesAdaptive Teaching Guide: Private Education Assistance CommitteeMarvin MoreteNo ratings yet
- Inbound 2665093207043498969Document2 pagesInbound 2665093207043498969bvcxd7m4j4No ratings yet
- Vigorous Lesson Plan SampleDocument6 pagesVigorous Lesson Plan Sampleapi-532406115No ratings yet
- Topic 4 BondingDocument13 pagesTopic 4 Bondinglobna masadehNo ratings yet
- DP Chem Unit 4 Chemical Bonding and StructuresDocument7 pagesDP Chem Unit 4 Chemical Bonding and StructuresPatrick AbidraNo ratings yet
- LP 17 Polarity of A Molecule Based On Its ElectronegativityDocument2 pagesLP 17 Polarity of A Molecule Based On Its ElectronegativityKeeyt KytheNo ratings yet
- Physical Science DLP Q1W2Document7 pagesPhysical Science DLP Q1W2junar asentista50% (2)
- Lesson Plan For ELECTRONEGATIVITYDocument7 pagesLesson Plan For ELECTRONEGATIVITYHAROLD PAYUNAN100% (1)
- DLL Chem Dec02Document4 pagesDLL Chem Dec02Rosallie Caaya-NuezNo ratings yet
- PHYSICAL SCIENCE Q3 Week 2 - v2Document31 pagesPHYSICAL SCIENCE Q3 Week 2 - v2Evangelyn Patatag-CatacutanNo ratings yet
- Physical Science DLP Q1W2Document7 pagesPhysical Science DLP Q1W2JennyMaeAguilarMeruNo ratings yet
- Science 9 - Q2 - Week2 - Melc 5Document10 pagesScience 9 - Q2 - Week2 - Melc 5Rhyan Zero-four BaluyutNo ratings yet
- Week 3 - LeDocument10 pagesWeek 3 - LeRodney BarbaNo ratings yet
- LESSON PLAN-2-Covalent BondingDocument2 pagesLESSON PLAN-2-Covalent BondingHOWARD ZULUNo ratings yet
- Bonding and Structure Transition Guide Checkpoint TaskDocument9 pagesBonding and Structure Transition Guide Checkpoint TaskJustjesting1460No ratings yet
- Lesson Plan: Lesson: Further Covalent BondingDocument6 pagesLesson Plan: Lesson: Further Covalent BondingMarcTnnNo ratings yet
- Module 2 Chemical Bonding and The Shapes of MoleculesDocument30 pagesModule 2 Chemical Bonding and The Shapes of MoleculesJulius Gutierrez EngbinoNo ratings yet
- Planning Sheet For Single Science Lesson Lesson Title: "The Name Is Bond. Covalent Bond." Cluster: Chemistry in Action S.L.O:S2-2-02 Grade: 10Document4 pagesPlanning Sheet For Single Science Lesson Lesson Title: "The Name Is Bond. Covalent Bond." Cluster: Chemistry in Action S.L.O:S2-2-02 Grade: 10Eunice CorreaNo ratings yet
- Lesson Plan Ionic Bonding - LessonDocument6 pagesLesson Plan Ionic Bonding - LessonCristina Nicomedes AguinaldoNo ratings yet
- Lesson Plan: Purpose of The Lesson) by The End of The Lesson, The Learners Would Be Able ToDocument5 pagesLesson Plan: Purpose of The Lesson) by The End of The Lesson, The Learners Would Be Able ToNJABULO NGUBANENo ratings yet
- Chapter 6 Chemical Bonding HomeworkDocument5 pagesChapter 6 Chemical Bonding Homeworkafodegeydsqpga100% (1)
- Grade 9 Daily Lesson Plan: Viga Rural Development High School 9 Science Date Section Time 2nd Quarter I. ObjectivesDocument2 pagesGrade 9 Daily Lesson Plan: Viga Rural Development High School 9 Science Date Section Time 2nd Quarter I. ObjectivesSaudia Julia Singzon100% (1)
- Lesson Plan: Content Area Topic/Concept/Ski LLDocument4 pagesLesson Plan: Content Area Topic/Concept/Ski LLerum khanNo ratings yet
- SHS Physical Science Q1 SLM - 3Document25 pagesSHS Physical Science Q1 SLM - 3Adalee ColleenNo ratings yet
- DLL Chem Gr9 Covalent BondDocument4 pagesDLL Chem Gr9 Covalent BondxoxkakidoxoxNo ratings yet
- LP1 Gen. Chemistry 2 3rd Quarter SY2022-2023 CheckedDocument17 pagesLP1 Gen. Chemistry 2 3rd Quarter SY2022-2023 CheckedLady mistressNo ratings yet
- Lesson Plan 380Document13 pagesLesson Plan 380api-583311172No ratings yet
- Ity of Simple Compounds and Molecules - v4 Nikki BornalesDocument15 pagesIty of Simple Compounds and Molecules - v4 Nikki Bornalestvwolf332No ratings yet
- daily-Lesson-Plan-for - PhysScie-Vsepr-TheoryDocument9 pagesdaily-Lesson-Plan-for - PhysScie-Vsepr-TheoryMaria CongNo ratings yet
- Chemistry S4 SBDocument461 pagesChemistry S4 SBumulisagerardine123No ratings yet
- Sample LE PS W3 ADocument8 pagesSample LE PS W3 AJoseph GutierrezNo ratings yet
- Chapter 2: The Chemical Context of LifeDocument11 pagesChapter 2: The Chemical Context of LifeDan JohnsonNo ratings yet
- 0893 Intro OTG Week2.3 SOW+Extract+Stage+9Document7 pages0893 Intro OTG Week2.3 SOW+Extract+Stage+9Zon PhooNo ratings yet
- A Lesson Plan in Chemical BondingDocument5 pagesA Lesson Plan in Chemical BondingEllen Grace Dela PeñaNo ratings yet
- G9 SLEM Q2 W2 Ionic Covalent PropertiesDocument17 pagesG9 SLEM Q2 W2 Ionic Covalent PropertiesStephanie Villanueva100% (1)
- Lesson Plan: Lesson: Rate of ReactionDocument6 pagesLesson Plan: Lesson: Rate of ReactionMarcTnnNo ratings yet
- Grade 9 - 2nd Quarter - Week 8 Day 3 - 4Document17 pagesGrade 9 - 2nd Quarter - Week 8 Day 3 - 4zero kakumaruNo ratings yet
- Siop Lesson Chemical BondingDocument3 pagesSiop Lesson Chemical Bondingapi-423859884No ratings yet
- Grade 9 - 2ND Quarter - Week 8 Day 3 - 4Document17 pagesGrade 9 - 2ND Quarter - Week 8 Day 3 - 4Marjohn ElentorioNo ratings yet
- Outcomes From Alberta Program of StudiesDocument4 pagesOutcomes From Alberta Program of Studiesapi-266431226No ratings yet
- CHEM 1 MODULE 6 (Covalent Bonding)Document6 pagesCHEM 1 MODULE 6 (Covalent Bonding)Joseph ZafraNo ratings yet
- Unit 3 Grade 9 Sept 09Document28 pagesUnit 3 Grade 9 Sept 09girmaamanNo ratings yet
- Science 9 q2 Mod2Document16 pagesScience 9 q2 Mod2Prince U KennardNo ratings yet
- q1l3 - 2nd Sem Physical ScienceDocument12 pagesq1l3 - 2nd Sem Physical ScienceBilly Jasper DomingoNo ratings yet
- Atg Met 1 Lesson 2 ImfDocument5 pagesAtg Met 1 Lesson 2 ImfJessanin Pabriga IINo ratings yet
- Quarter 2 Countless and Active Particles of Matter: Learner's Activity SheetDocument8 pagesQuarter 2 Countless and Active Particles of Matter: Learner's Activity SheetHersheyNo ratings yet
- Bond PredictionlessonDocument9 pagesBond PredictionlessonDerrick MNo ratings yet
- Bond - Chemical Bond (10th-11th Grade)Document42 pagesBond - Chemical Bond (10th-11th Grade)jv peridoNo ratings yet
- Lesson Plan For Vsepr TheoryDocument8 pagesLesson Plan For Vsepr TheoryAileen gay PayunanNo ratings yet
- Chemical Bonding Lesson PlanDocument3 pagesChemical Bonding Lesson PlanGabriel ClaverieNo ratings yet
- Core-Physical Science Q1 SLM - 5Document17 pagesCore-Physical Science Q1 SLM - 5Christopher Agustin Tambogon Lpt100% (1)
- Genchem 1 DLPDocument7 pagesGenchem 1 DLPDhevin VergaraNo ratings yet
- The New Chemist Company Publications- Accessible Organic Chemistry: The New Chemist CompanyFrom EverandThe New Chemist Company Publications- Accessible Organic Chemistry: The New Chemist CompanyNo ratings yet
- Exercise 3Document6 pagesExercise 3Bank Yossy WoluslaweNo ratings yet
- (Untuk Siswa) Kisi-Kisi Soal PSSP SMP NSA 23-24 (Chemistry)Document2 pages(Untuk Siswa) Kisi-Kisi Soal PSSP SMP NSA 23-24 (Chemistry)Bank Yossy WoluslaweNo ratings yet
- Pas GR11 Question Finalterm Odd (I) SMTR 23-24Document6 pagesPas GR11 Question Finalterm Odd (I) SMTR 23-24Bank Yossy WoluslaweNo ratings yet
- Igcse Chemistry 5ed TR Eoc Test Answers 15Document1 pageIgcse Chemistry 5ed TR Eoc Test Answers 15Bank Yossy Woluslawe100% (1)
- Practice Chemistry Final Test Grade 8Document2 pagesPractice Chemistry Final Test Grade 8Bank Yossy WoluslaweNo ratings yet
- Atoms and Molecules 2Document50 pagesAtoms and Molecules 2Bank Yossy WoluslaweNo ratings yet
- Igcse Chemistry 5ed TR Eoc Test Answers 16Document1 pageIgcse Chemistry 5ed TR Eoc Test Answers 16Bank Yossy WoluslaweNo ratings yet
- Rate of Reaction Worksheet - Effervescent TabletDocument3 pagesRate of Reaction Worksheet - Effervescent TabletBank Yossy WoluslaweNo ratings yet
- Aileen-Pptx - 20230321 - Aileen EditedDocument10 pagesAileen-Pptx - 20230321 - Aileen EditedBank Yossy WoluslaweNo ratings yet
- Analisis PTS 11 IPA 2 - 2324Document13 pagesAnalisis PTS 11 IPA 2 - 2324Bank Yossy WoluslaweNo ratings yet
- Eoc - Test - Chapter - 15 & 16Document2 pagesEoc - Test - Chapter - 15 & 16Bank Yossy WoluslaweNo ratings yet
- Module As Level ChemistryDocument407 pagesModule As Level ChemistryBank Yossy WoluslaweNo ratings yet
- Distillation Worksheet What Is Distillation?Document2 pagesDistillation Worksheet What Is Distillation?Bank Yossy WoluslaweNo ratings yet
- Igcse Coordinated Sciences 0654 Combined Science 0 - 59cc8a591723ddab3bbdfff3 PDFDocument50 pagesIgcse Coordinated Sciences 0654 Combined Science 0 - 59cc8a591723ddab3bbdfff3 PDFBank Yossy WoluslaweNo ratings yet
- Budgeting Sponsorship STAR CARNIVALDocument1 pageBudgeting Sponsorship STAR CARNIVALBank Yossy WoluslaweNo ratings yet
- NeutralisationDocument12 pagesNeutralisationBank Yossy WoluslaweNo ratings yet
- Igcse Coordinated Sciences 0654 Combined Science 0 - 59cc8a591723ddab3bbdfff3 PDFDocument50 pagesIgcse Coordinated Sciences 0654 Combined Science 0 - 59cc8a591723ddab3bbdfff3 PDFBank Yossy WoluslaweNo ratings yet
- Allow Me To Introduce MyselfDocument1 pageAllow Me To Introduce MyselfBank Yossy WoluslaweNo ratings yet
- Basic Course Outline (Bco)Document7 pagesBasic Course Outline (Bco)Bank Yossy WoluslaweNo ratings yet
- Understanding Auto, Optical and Metrics Kerning in Adobe IllustratorDocument5 pagesUnderstanding Auto, Optical and Metrics Kerning in Adobe Illustratorsabaorosa8832No ratings yet
- Dinathanthi 2013 Putthandu PalangalDocument24 pagesDinathanthi 2013 Putthandu PalangalViveha AnandhanNo ratings yet
- Contemporary Egyptian LiteratureDocument16 pagesContemporary Egyptian LiteraturebascutaNo ratings yet
- PK 3Document9 pagesPK 32021mec.r47No ratings yet
- Social Science PreboardDocument1 pageSocial Science PreboardBhavya DNo ratings yet
- Circular No:7 Date: 22 June 2021 Subject: Internal Assessment: Grade 8Document10 pagesCircular No:7 Date: 22 June 2021 Subject: Internal Assessment: Grade 8aneesshkNo ratings yet
- Razorlabs Ovh-3Document9 pagesRazorlabs Ovh-3obsdcloudNo ratings yet
- Handy MySQL CommandsDocument6 pagesHandy MySQL CommandsKatherine JamesNo ratings yet
- Line Meaning A Red, Red Rose AnalysisDocument2 pagesLine Meaning A Red, Red Rose AnalysisPutri IntanNo ratings yet
- Cambridge International AS & A Level: English Language 9093/13Document15 pagesCambridge International AS & A Level: English Language 9093/13Karma RouaidyNo ratings yet
- C.E.G.H.I.K. Ang Pista NG Sambayanan (Responses)Document10 pagesC.E.G.H.I.K. Ang Pista NG Sambayanan (Responses)MickJagger Ronio100% (2)
- How To Create SDF FilesDocument2 pagesHow To Create SDF FilesSandra RodriguezNo ratings yet
- Big Data Solution Assignment-IDocument4 pagesBig Data Solution Assignment-INAGA KUMARI ODUGUNo ratings yet
- Systemic Functional Linguistics: The Perfect Match For Content and Language Integrated LearningDocument7 pagesSystemic Functional Linguistics: The Perfect Match For Content and Language Integrated LearningENG331C F21No ratings yet
- Tieng Anh 9 Số 7Document3 pagesTieng Anh 9 Số 7Huyền TrầnNo ratings yet
- Mathematics For Physicist: Adhi Harmoko SaputroDocument68 pagesMathematics For Physicist: Adhi Harmoko SaputroMila AprianiNo ratings yet
- Celebrity Constellation Cruise Ship Itineraries 2020-2021 0Document4 pagesCelebrity Constellation Cruise Ship Itineraries 2020-2021 0Fahmi Bango BeracunNo ratings yet
- Unit 5 Quantifiers Grammar C.CDocument2 pagesUnit 5 Quantifiers Grammar C.Chbat saidNo ratings yet
- The Last Lesson Important Questions CBSE Class 12 EnglishDocument18 pagesThe Last Lesson Important Questions CBSE Class 12 EnglishDisha ChawlaNo ratings yet
- PHILOKALIA, Vol-4, Malayalam PDFDocument713 pagesPHILOKALIA, Vol-4, Malayalam PDFbengo80% (5)
- Order Management ProjectDocument18 pagesOrder Management ProjectmarcialefrediriqueNo ratings yet
- Kalaha in Java - An Experiment in Artificial Intelligence and NetworkingDocument102 pagesKalaha in Java - An Experiment in Artificial Intelligence and NetworkingapNo ratings yet
- Junos OverviewDocument451 pagesJunos OverviewDanarNo ratings yet
- SS7MD PMDocument191 pagesSS7MD PMAkhil GuptaNo ratings yet
- Region VII EASTERN VISAYASDocument7 pagesRegion VII EASTERN VISAYASAirah cayetanoNo ratings yet
- Q1W2 Media Information Technology LiteracyDocument11 pagesQ1W2 Media Information Technology LiteracyDaisiree PascualNo ratings yet
- Civil Enlil NinlilDocument25 pagesCivil Enlil NinlileannatumNo ratings yet
- Human Genetics 10th Edition Lewis Test BankDocument25 pagesHuman Genetics 10th Edition Lewis Test BankAnthonyWilliamsbptf100% (53)
- Laboaratory Report: Lab Excersice 4: Standard Sequential CircuitsDocument12 pagesLaboaratory Report: Lab Excersice 4: Standard Sequential CircuitsDiego OrtegaNo ratings yet
- Linking Novas Files With Simulators and Enabling FSDB DumpingDocument134 pagesLinking Novas Files With Simulators and Enabling FSDB Dumpinghdpisces88% (8)