Professional Documents
Culture Documents
5.60 Thermodynamics & Kinetics: Mit Opencourseware
5.60 Thermodynamics & Kinetics: Mit Opencourseware
Uploaded by
Eduardo GomezOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
5.60 Thermodynamics & Kinetics: Mit Opencourseware
5.60 Thermodynamics & Kinetics: Mit Opencourseware
Uploaded by
Eduardo GomezCopyright:
Available Formats
MIT OpenCourseWare
http://ocw.mit.edu
5.60 Thermodynamics & Kinetics
Spring 2008
For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.
5.60 Spring 2008
Lecture #3
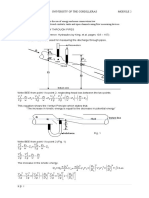
Isothermal Gas Expansion
page 1
(T = 0)
gas (p1, V1, T) = gas (p2, V2, T)
Irreversibly (many ways possible)
(1)
Set pext = 0
p= 0
T
p= 0
p 1,V1
p 2,V2
v2
w (1) = pext dV = 0
V1
(2)
Set pext = p2
p2
T
p2
T
p 2,V2
p 1,V1
v2
w (2) = p2dV = p2 (V2 V1 )
V1
p
p1
p2
V1
-w(2)
V2
Note, work is negative: system expands against surroundings
5.60 Spring 2008
(3)
Lecture #3
page 2
Carry out change in two steps
gas (p1, V1, T) = gas (p3, V3, T) = gas (p2, V2, T)
p1 > p3 > p2
p2
p3
p3
T
p 2,V2
p 3,V3
p 1,V1
v3
v2
V1
V3
w (3) = p3dV p2dV = p3 (V3 V1 ) p2 (V2 V3 )
p
p1
More work delivered to
surroundings in this case.
p3
p2
V1 V3
V2
-w(3)
(4)
Reversible change
p = pext throughout
V
wrev = 2 pdV
V1
p1
p2
V1
V2
rev
For ideal gas:
V
wrev = 2
V1
Maximum work delivered to
surroundings for isothermal gas
expansion is obtained using a
reversible path
V
p
nRT
dV = nRT ln 2 = nRT ln 2
V
V1
p1
5.60 Spring 2008
Lecture #3
page 3
The Internal Energy U
dU = d-q + d-w
(First Law)
dU = C pathdT pext dV
And U (T ,V
U
U
dU =
dT +
dV
Some frequent constraints:
dU = d-qrev + d-wrev = d-qrev pdV
Reversible
Isolated
d-q = d-w = 0
Adiabatic
d-q = 0
Constant V
(p = pext )
reversible
dU = d-w =
-pdV
w = 0 dU = d-qV
Constant V
U
U
dU =
dT +
dV
T V
V T
but also
U
d-qV =
dT
T V
d-qV = CV dT
So
= CV
T V
very important result!!
V T
dU = CV dT +
dV
what is this?
5.60 Spring 2008
Lecture #3
U
)
V T
Joule Free Expansion of a Gas
gas
(to get
vac
gas (p1, T1, V1) = gas (p2, T2, V2)
Since q = w = 0
Recall
Adiabatic
q=0
Expansion into Vac.
(pext=0)
w=0
dU or U = 0
Constant U
U
dV = 0
V T
dU = CV dT +
U
V T
dVU = CV dTU
= CV
V T
Joule did this.
page 4
V U
measure in Joule exp't!
V U
T
T
dU = CV dT CV J dV
=
J
V U V U
Joule coefficient
lim
V 0
For Ideal gas
J = 0
dU = CV dT
U(T)
exactly
Always for ideal gas
only depends on T
The internal energy of an ideal gas depends only on temperature
Consequences
U = 0
U = CV dT
For all isothermal expansions or
compressions of ideal gases
For any ideal gas change in state
You might also like
- ME2121 Finals ReviewDocument4 pagesME2121 Finals ReviewSherman LiamNo ratings yet
- Cooling TowerDocument23 pagesCooling TowerBevelyn L. Barreto HernandezNo ratings yet
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Isothermal Gas Expansion: P V T P V TDocument4 pagesIsothermal Gas Expansion: P V T P V Twolfofphysics08IPMP01No ratings yet
- Thermodynamic Lesson 2Document7 pagesThermodynamic Lesson 2kelebekkNo ratings yet
- Expansions, Energy, Enthalpy Isothermal Gas Expansion: P V T P V TDocument7 pagesExpansions, Energy, Enthalpy Isothermal Gas Expansion: P V T P V TNaveen NaviNo ratings yet
- Sasi Institute of Technology EnineeringDocument6 pagesSasi Institute of Technology EnineeringHamu NalaNo ratings yet
- ProcessDocument5 pagesProcessNarcisse Serge NouadjepNo ratings yet
- Solution#1 PDFDocument4 pagesSolution#1 PDFuchnnaNo ratings yet
- (Physics, Thermal) - Solutions To Statistical Mechanics ProblemsDocument7 pages(Physics, Thermal) - Solutions To Statistical Mechanics ProblemsabartoskiNo ratings yet
- First Law (Contd.) : P V WorkDocument5 pagesFirst Law (Contd.) : P V Workashish44571No ratings yet
- Chapter 2 FormulasDocument6 pagesChapter 2 FormulasShellyNo ratings yet
- ThermodynamicsDocument9 pagesThermodynamicssamir boseNo ratings yet
- Example-Water Flow in A PipeDocument13 pagesExample-Water Flow in A PipecristinelbNo ratings yet
- Chapter 6Document11 pagesChapter 6Analie Buerano SagunNo ratings yet
- Thermodynamics Intro and The First LawDocument17 pagesThermodynamics Intro and The First Lawrin rinNo ratings yet
- BIS 154 - Mech Eng. 2 - Lecture 3.ppsxDocument32 pagesBIS 154 - Mech Eng. 2 - Lecture 3.ppsxMohamed NadaNo ratings yet
- Unit 2 First Law-Closed System ProblemsDocument11 pagesUnit 2 First Law-Closed System Problemspiravi66No ratings yet
- Coupling of Elasticity, Flow and Material BalanceDocument29 pagesCoupling of Elasticity, Flow and Material BalanceDicky AlviansyahNo ratings yet
- Report-Open-Hydraulics-Energy EquationDocument8 pagesReport-Open-Hydraulics-Energy EquationJohn Eric CabañaNo ratings yet
- 351 F 22 Exam EquationsDocument1 page351 F 22 Exam EquationsEdaNo ratings yet
- Chapter 2 BDocument11 pagesChapter 2 BStefanPerendijaNo ratings yet
- Thermodynamics Formulae BookletDocument2 pagesThermodynamics Formulae BookletwardeqNo ratings yet
- RTT Mass, EnergyDocument5 pagesRTT Mass, EnergyPatrick Joseph RoblesNo ratings yet
- Lecture 2 EDocument8 pagesLecture 2 EMihai MirceaNo ratings yet
- Q W U H S: Isotérmico DT 0 Isobárico DP 0 Isocórico DV 0 Adiabático DQ 0Document2 pagesQ W U H S: Isotérmico DT 0 Isobárico DP 0 Isocórico DV 0 Adiabático DQ 0Aldasaurio SPNo ratings yet
- Physical Work 3Document25 pagesPhysical Work 3ScribdTranslationsNo ratings yet
- 102MAE Thermodynamics Formula SheetDocument2 pages102MAE Thermodynamics Formula SheetBogdan ProfirNo ratings yet
- Thermodynamics FormulaDocument9 pagesThermodynamics FormulaJayvie TumangNo ratings yet
- Chapter 3 - Section B - Non-Numerical SolutionsDocument12 pagesChapter 3 - Section B - Non-Numerical SolutionsAwaludin R FirmanshahNo ratings yet
- Ayuda para Balance de Masa y MomentumDocument4 pagesAyuda para Balance de Masa y MomentumalvaroNo ratings yet
- HW5 SolDocument3 pagesHW5 SoloppipxNo ratings yet
- Physics - Solution Set For Homework Book Chapter - 15 KTG & ThermodynamicsDocument4 pagesPhysics - Solution Set For Homework Book Chapter - 15 KTG & ThermodynamicsKishorNo ratings yet
- Equations FinalExamDocument5 pagesEquations FinalExamel locassinNo ratings yet
- Compressible Flow Through Nozzles and Diffusers: V DT V D V VDocument14 pagesCompressible Flow Through Nozzles and Diffusers: V DT V D V VCamilo SantacruzNo ratings yet
- Thermodynamic Processes and DerivationDocument10 pagesThermodynamic Processes and DerivationAbenayaNo ratings yet
- Formulário - Mecânica Dos Fluidos (MEE210/MEE620/MEE630)Document2 pagesFormulário - Mecânica Dos Fluidos (MEE210/MEE620/MEE630)Victor TavaresNo ratings yet
- Αντστροφείς MosDocument37 pagesΑντστροφείς MosvinothrathinamNo ratings yet
- Formelsammlung - ZustandsänderungenDocument2 pagesFormelsammlung - ZustandsänderungenTrần Nguyên KhôiNo ratings yet
- Formula Sheet + Charts Must Be Returned A3er The ExamDocument19 pagesFormula Sheet + Charts Must Be Returned A3er The ExamRaphael ErfeNo ratings yet
- Termodinamica ch03Document35 pagesTermodinamica ch03Rebeca AlmeidaNo ratings yet
- 7B Final Equation SheetDocument3 pages7B Final Equation SheetFangZiWenNo ratings yet
- Physics 112 Formulae IIIDocument3 pagesPhysics 112 Formulae IIISaied RajehaNo ratings yet
- Lecture 12+13 - Transient Waves On Transmission LinesDocument11 pagesLecture 12+13 - Transient Waves On Transmission Linessamer saeedNo ratings yet
- Allowed Cheat SheetDocument2 pagesAllowed Cheat SheetNguyễn Tương QuỳnhNo ratings yet
- Transmission Line Different Types of Transmission Line Transmission Line Per Unit Length Parameters Telegrapher Equation Power FlowDocument15 pagesTransmission Line Different Types of Transmission Line Transmission Line Per Unit Length Parameters Telegrapher Equation Power FlowdiptodevilNo ratings yet
- Total Differential, Rates of Change and Small ChangesDocument6 pagesTotal Differential, Rates of Change and Small ChangesChainarong TaepanichNo ratings yet
- Hydro 1 Module 2Document13 pagesHydro 1 Module 2Jericho Alfred Rullog Sapitula100% (1)
- Fluid Mechanics I Solution 4 Question 1: Problem P2.139: A B B C D DDocument8 pagesFluid Mechanics I Solution 4 Question 1: Problem P2.139: A B B C D Dcartoon_nateNo ratings yet
- Problem 4.158Document2 pagesProblem 4.158문jmtNo ratings yet
- Can IdealDocument3 pagesCan IdealHERRERA GAVINO LORIAN GERMANNo ratings yet
- PETE 411 Well Drilling: Surge and Swab PressuresDocument40 pagesPETE 411 Well Drilling: Surge and Swab PressuresBendali MehdiNo ratings yet
- Ideal GasDocument1 pageIdeal GasMike Raphy T. VerdonNo ratings yet
- Quiz 1 - MA SolutionsDocument6 pagesQuiz 1 - MA SolutionsabullaNo ratings yet
- Solución Parcial Mecánica de FluidosDocument5 pagesSolución Parcial Mecánica de FluidosIñigoNo ratings yet
- 20 562ln08Document8 pages20 562ln08sammy wanakaiNo ratings yet
- Compressibility PDFDocument2 pagesCompressibility PDFJuan Daniel CabreraNo ratings yet
- Finite Volume Method For One DimensionalDocument26 pagesFinite Volume Method For One Dimensionalscience1990No ratings yet
- A V P A V P: PressureDocument2 pagesA V P A V P: PressurePearl Alexandra FabitoNo ratings yet
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99From EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99No ratings yet
- Ishrae 365 2009 PDFDocument16 pagesIshrae 365 2009 PDFZeeshan HasanNo ratings yet
- HumidificationDocument32 pagesHumidificationTanvir AhmedNo ratings yet
- Automotive Air Condition and Climate ControlsDocument2 pagesAutomotive Air Condition and Climate ControlsrhonieventuracvsuNo ratings yet
- Thermodynamic Real-Life SamplesDocument2 pagesThermodynamic Real-Life SamplesKaren SargentoNo ratings yet
- 11.2 Expansion Tank: Chapter 11 Heating - Hydronic System SizingDocument2 pages11.2 Expansion Tank: Chapter 11 Heating - Hydronic System SizingHussam AgabNo ratings yet
- Compression Process CalculationsDocument2 pagesCompression Process CalculationsRahul ChandrawarNo ratings yet
- Example CH 4Document4 pagesExample CH 4Uday Prakash SahuNo ratings yet
- Lab 8Document5 pagesLab 8huzaifa zainNo ratings yet
- HVAC EconomizerDocument14 pagesHVAC EconomizerShoukat Ali ShaikhNo ratings yet
- Temperature Conversion WorksheetDocument1 pageTemperature Conversion WorksheetEJ DelimaNo ratings yet
- Chapter 2 (Numerical+solution)Document60 pagesChapter 2 (Numerical+solution)Sudeep magarNo ratings yet
- Fluid DynamicsDocument4 pagesFluid DynamicsAidilmann AshrafeiderNo ratings yet
- The Essentials of Chilled Beams - Part 2: Skills WorkshopDocument3 pagesThe Essentials of Chilled Beams - Part 2: Skills WorkshoprkibNo ratings yet
- Psychrometry ProblemsDocument33 pagesPsychrometry Problems19R21A0334 MATTA SHASHANKNo ratings yet
- Uigi SiDocument1 pageUigi SiArikah MukasyafahNo ratings yet
- PSYCHRODocument11 pagesPSYCHROMrinal Kanti BhaduriNo ratings yet
- OAU Rev1Document5 pagesOAU Rev1Tassawac Koeiop100% (1)
- Test Bank Ch07Document10 pagesTest Bank Ch07Kagiso MokalakeNo ratings yet
- Psychrometric ChartsDocument12 pagesPsychrometric ChartsDhanyaUnniNo ratings yet
- ME 441-NotesDocument4 pagesME 441-NotesAshish ManwarNo ratings yet
- Lab 7Document2 pagesLab 7Nadia NasirNo ratings yet
- Ref Systems Compiled ProblemsDocument2 pagesRef Systems Compiled ProblemsMartillano, Oliver C.No ratings yet
- Thermowell Calculation GuideDocument19 pagesThermowell Calculation Guideprasanthk22No ratings yet
- Cabinet Cooler System Sizing GuideDocument1 pageCabinet Cooler System Sizing GuideAlexis BerrúNo ratings yet
- Chapter 7 PDFDocument46 pagesChapter 7 PDFOnur ÖZÇELİKNo ratings yet
- Assignment 3Document19 pagesAssignment 3ashna latheefNo ratings yet
- Triple Point of Water - AssignmentDocument13 pagesTriple Point of Water - AssignmentpatilsspNo ratings yet
- Catalogo CiiDocument2 pagesCatalogo CiiSergio ChupinaNo ratings yet
- En J02.DAI.74 Daikin RZASG M Technical Data RZASG MV1 Data BookDocument49 pagesEn J02.DAI.74 Daikin RZASG M Technical Data RZASG MV1 Data BookNuno MotaNo ratings yet