Professional Documents
Culture Documents
(Pply) .+xli2: Kerosene Air T
(Pply) .+xli2: Kerosene Air T
Uploaded by
Javier Rojas PaytanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
(Pply) .+xli2: Kerosene Air T
(Pply) .+xli2: Kerosene Air T
Uploaded by
Javier Rojas PaytanCopyright:
Available Formats
46
CHAPTER 2
Working in terms of absolute pressure heads, [(pA),+ 8581(0.100)/[(0.90)(9.79)1 2.90 =
(160)(0.100)/[(0.90)(9.79)], (pA)= 442 mbar; [(-442 + 858)(0.100)]/[(0.90)(9.79)] + (2.10 + z) (z)
(13.6/0.90) = 0, z = 0.483 m.
2.70
A pipeline contains an incompressible gas (y = 0.05 lb/ft3) at rest; at point A the pressure is 4.69 in of water.
What is the pressure, in inches of water, at point B, 492 ft higher than A?
The change in pressure in the atmosphere must be considered; assume, however, that yair = 0.076 lb/ft3 is
constant.
(PA/ Y).b. = (PA/Y)m. + 4.69/12 ft of water
(1)
(PB/Y)abs = (Pply).+xli2 ft of water
(2)
(PRI Y)abs = (PAIY).t. (PBIY)..+ 4.69/12 x/12
(3)
Subtracting Eq. (2) from Eq. (1),
(PAI
(pAlr). (p./y).,= 492 ft of air = (492)(0.076/62.4) = 0.599 ft of water
(pAly).b. (p.ly).,,s= 492 ft of gas = (492)(0.05/62.4) = 0.394 ft of water
Substituting these relationships into Eq. (3), 0.394 = 0.599 + 4.69/12 x/12, x = 7.15 in of water.
2.71
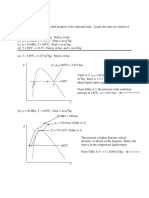
Determine the pressure difference between points A and B in Fig. 2-51.
PA + [(0.88)(9.79)](0.21) [(13.6)(9.79)](0.09) [(0.82)(9.79)1(0.41 0.09)
+ (9.79)(0.41 0.15) (0.0118)(0.10) = pB
PA PB = 10.2 kPa
-
Kerosene
Air
T
9 _ L 1 5
c m
41
c m
10cm
Benzene
Mercury
2.72
Fig. 2-51
Water
In Fig. 2-52, if pB pA = 97.4 kPa, calculate H.
PA (9.79)(H/100) R0.827)(9.79)1(14) + [(13.6)(9.79)][(34 + H 17)1100] =PB
1.234H + 66.53 = !)B PA= 97.4
Meriam
red oil,
s.g. = 0.827
H = 25.0 cm
17c
m
W a t e r
Mercury
34cm
Fig. 2-52
You might also like
- Pipe Size CalculationDocument5 pagesPipe Size CalculationChristoberNo ratings yet
- PPE Problem Set 1Document128 pagesPPE Problem Set 1Gracee86% (14)
- Design Considerations For Pools and Spas (Natatoriums) : John W. Lund Geo-Heat CenterDocument3 pagesDesign Considerations For Pools and Spas (Natatoriums) : John W. Lund Geo-Heat CenterНебојша РадићNo ratings yet
- MonevaDocument15 pagesMonevaKurt Lester Moneva100% (1)
- CompreDocument9 pagesCompreLara GatbontonNo ratings yet
- Fluids HW SolutionDocument8 pagesFluids HW SolutionDennis Brown100% (1)
- 1 Practice Problems (2 - 2)Document3 pages1 Practice Problems (2 - 2)IAN PAOLO BAUTISTA100% (1)
- Fluid MechanicsDocument6 pagesFluid MechanicsPAULINO ALCARAZ JRNo ratings yet
- Fluid MechanicsDocument6 pagesFluid MechanicsLorde Wagayen100% (1)
- Chapter 3 0114Document15 pagesChapter 3 0114Cyduck Guevarra100% (2)
- Nozzle EquationsDocument8 pagesNozzle EquationsStellin AmodraNo ratings yet
- Exercise 1 Sayas Ej2Document5 pagesExercise 1 Sayas Ej2bryan50% (2)
- Chapter 12 - ThermodynamicsDocument69 pagesChapter 12 - Thermodynamicszahid_polyNo ratings yet
- Absorption NotesDocument79 pagesAbsorption Noteshanisshi100% (2)
- ESAS Actual Board. Thank Me LaterDocument5 pagesESAS Actual Board. Thank Me LaterRhea Pardo PeralesNo ratings yet
- Sample Problem ThermoDocument25 pagesSample Problem ThermoJonnah Faye Mojares0% (1)
- THERMODYNAMICS - MODULE 2 - Lesson 4 5 - Week 8 11 - As of Nov 4Document42 pagesTHERMODYNAMICS - MODULE 2 - Lesson 4 5 - Week 8 11 - As of Nov 4Kim OpenaNo ratings yet
- Fluid Statics PDFDocument28 pagesFluid Statics PDFAnthony Leire MontealtoNo ratings yet
- Tutorial Chapter 02 - AnswerDocument8 pagesTutorial Chapter 02 - AnswerFateh Hakeem100% (4)
- Solution Manual Fluid Mechanics 4th Edition Frank M WhiteDocument17 pagesSolution Manual Fluid Mechanics 4th Edition Frank M WhiteDievo Neimhpulov67% (3)
- 2 Hydrostatics ContinuationDocument5 pages2 Hydrostatics ContinuationPritz Jay Magno TorresNo ratings yet
- Hydraulics - Topic 2 - Continuation - 26 May 2022Document27 pagesHydraulics - Topic 2 - Continuation - 26 May 2022samera salihNo ratings yet
- ETDocument525 pagesETAshok GovindharasuNo ratings yet
- Fluid ReferenceDocument19 pagesFluid ReferencemarkalvinbonNo ratings yet
- HIDROSTATIC (Question)Document4 pagesHIDROSTATIC (Question)kechik_bollockNo ratings yet
- HYDRAULICS TOPIC 1 FLUID PROPERTIES UNIT PRESSURE 23 May 2022Document41 pagesHYDRAULICS TOPIC 1 FLUID PROPERTIES UNIT PRESSURE 23 May 2022samera salihNo ratings yet
- 4 Bohan ADocument2 pages4 Bohan AJhenny GuayguaNo ratings yet
- Fluid Mechanics Exercices Enstab Cou SWDocument12 pagesFluid Mechanics Exercices Enstab Cou SWLourenze KigisNo ratings yet
- JuasDocument49 pagesJuasAndrés RodríguezNo ratings yet
- Converting Pump Head To PressureDocument5 pagesConverting Pump Head To PressuregoutamdeNo ratings yet
- Chapter TWODocument29 pagesChapter TWOMehdi BeyraktarNo ratings yet
- Y/G 0.01435 Ii) 1/0.00435 230: 0.14/32.2 SLNG/FTJ V, FtlslugDocument1 pageY/G 0.01435 Ii) 1/0.00435 230: 0.14/32.2 SLNG/FTJ V, FtlslugEdbert TulipasNo ratings yet
- 39Document1 page39Edbert TulipasNo ratings yet
- 478.8 2.11x10 1b 5m V P (0.99.8) (624) 1.09x10 Ft/sDocument1 page478.8 2.11x10 1b 5m V P (0.99.8) (624) 1.09x10 Ft/sEdbert TulipasNo ratings yet
- Linear InterpolationDocument6 pagesLinear Interpolationstephen jamesNo ratings yet
- Chapter 2Document5 pagesChapter 2Marco LuigiNo ratings yet
- Chapter 11Document30 pagesChapter 11younessinaNo ratings yet
- GasesDocument38 pagesGaseshNo ratings yet
- Problemas CompletosDocument16 pagesProblemas CompletosakksNo ratings yet
- Lecture 9Document3 pagesLecture 9Hassan OilNo ratings yet
- Me 211 Examples SolutionsDocument30 pagesMe 211 Examples SolutionsBryan Dominic Gabriel PaduaNo ratings yet
- Group Quiz Number 1 (Chapter 2 (Lecture1) ) .: 1) Complete The Table BelowDocument2 pagesGroup Quiz Number 1 (Chapter 2 (Lecture1) ) .: 1) Complete The Table BelowGab MercadoNo ratings yet
- HW3 SolutionDocument14 pagesHW3 SolutionBryceNo ratings yet
- Chem HelpDocument29 pagesChem HelpRobyn KentNo ratings yet
- ME262 Lecture2Document16 pagesME262 Lecture2FawadNo ratings yet
- 8echapter02 SMDocument115 pages8echapter02 SMAnonymous mXicTi8hBNo ratings yet
- MCQs Answers With Explanations U20AE301 AET SDocument127 pagesMCQs Answers With Explanations U20AE301 AET SGurunath AeroNo ratings yet
- Untitled 1Document3 pagesUntitled 1Jesica QuentNo ratings yet
- Thermo 5th Chap14 P115Document27 pagesThermo 5th Chap14 P115Pablo Isuart HdzNo ratings yet
- Thermodynamics - 1 Midterm SolutionDocument10 pagesThermodynamics - 1 Midterm SolutionEarl Maxie Lagdamin ErederaNo ratings yet
- Chapter 2: Pressure: ThermodynamicsDocument36 pagesChapter 2: Pressure: Thermodynamicsvipul100% (1)
- Realan GasDocument100 pagesRealan Gassedge54No ratings yet
- Chapter 2 - Tut-3Document5 pagesChapter 2 - Tut-3Anurag PanditNo ratings yet
- SolveDocument11 pagesSolveShamanAcolyteNo ratings yet
- ) : 3 - (Problems 8 Assignment No.: Solve The Following ProblemsDocument8 pages) : 3 - (Problems 8 Assignment No.: Solve The Following ProblemsMohNajiNo ratings yet
- Manual PDFDocument42 pagesManual PDFRyan MartinezNo ratings yet
- Xi - Cooling Tower - CheckDocument11 pagesXi - Cooling Tower - CheckJanine Glaiza Jaspe GallerosNo ratings yet
- Pa 1 /./ (DVLDR) F W Yv : Properties of Fluids 17 1.98Document2 pagesPa 1 /./ (DVLDR) F W Yv : Properties of Fluids 17 1.98Javier Rojas PaytanNo ratings yet
- 14 Q Chapter 1: DR (0.0402 - 0.0400) /2 0.0001 M A (70 (4.00/100) (4) 0.04398 MDocument2 pages14 Q Chapter 1: DR (0.0402 - 0.0400) /2 0.0001 M A (70 (4.00/100) (4) 0.04398 MJavier Rojas PaytanNo ratings yet
- h19,21 0 - / ' T - T y T,, / ,: MercurDocument2 pagesh19,21 0 - / ' T - T y T,, / ,: MercurJavier Rojas PaytanNo ratings yet
- A Prandtl's Soap-Film Analogy.: Properties of Fluids 0 19Document3 pagesA Prandtl's Soap-Film Analogy.: Properties of Fluids 0 19Javier Rojas PaytanNo ratings yet
- Gage 2 FTDocument2 pagesGage 2 FTJavier Rojas PaytanNo ratings yet
- Properties of Fluids: (P), F W Ma P mIVDocument1 pageProperties of Fluids: (P), F W Ma P mIVJavier Rojas PaytanNo ratings yet
- 94Document2 pages94Javier Rojas PaytanNo ratings yet
- AB. AB, 1 - FabDocument2 pagesAB. AB, 1 - FabJavier Rojas PaytanNo ratings yet
- Forces On Submerged Plane Areas 73Document2 pagesForces On Submerged Plane Areas 73Javier Rojas PaytanNo ratings yet
- A Bis A, H P/y H : Fluid Statics CL 29Document1 pageA Bis A, H P/y H : Fluid Statics CL 29Javier Rojas PaytanNo ratings yet
- 5.50 (A) (B) P Yh A - PR T: F 0 2T - 0 T AsteelasteelDocument1 page5.50 (A) (B) P Yh A - PR T: F 0 2T - 0 T AsteelasteelJavier Rojas PaytanNo ratings yet
- Forces On Submerged Curved Areas 107: 5.333ft 4 .3 3 1 FTDocument2 pagesForces On Submerged Curved Areas 107: 5.333ft 4 .3 3 1 FTJavier Rojas PaytanNo ratings yet