Professional Documents
Culture Documents
States of Matter: Acting Them Out: Summary: Science Content (2016 Curriculum)
Uploaded by
Renante CruzOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
States of Matter: Acting Them Out: Summary: Science Content (2016 Curriculum)
Uploaded by
Renante CruzCopyright:
Available Formats
States of matter: acting them out
Summary:
Students model the molecules in three states of matter, while seeing state changes in water.
Science content (2016 curriculum):
Chemistry: States of Matter, Properties of Materials (K-7)
Chemistry: Atoms, Molecules (3-7)
Lessons activity is in:
States of Matter in foods
States of Matter and State Changes
Materials:
Space for the students to move around in (classroom or outdoors)
Procedure:
Optional: start by reviewing the states of matter, while asking students to find examples in the
classroom. Solids have a fixed shape and are hard. Liquids can change shape, but always take up the
same amount of space (same volume) as they flow in their container. Gases change shape and fills
the space they are in (so change volume). Some students might mention plasma, the 4th state of
matter.
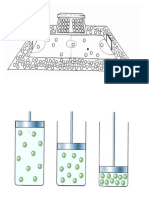
The particles (molecules or atoms) are arranged differently in different states of matter. Tell students
that they will model the particles in each of the three states of matter. They are each a molecule. Ask
them to stand in an open space of the classroom.
In a solid the molecules are packed tight, held together by strong bonds. Ask students to model a
solid by linking arms with their neighbours, so that they are packed tight together in a group. The
individuals can jiggle a little, but the group maintains its shape.
As a solid gains energy the molecules gain energy and can move around more. They are still bonded
to each other, but more loosely. Ask students to model a liquid by spreading apart and moving around
more, but always touching at least one other student with an outstretched arm. The bonds between
molecules break and form continuously, so that the group stays together but can move and change
shape, like a liquid.
As a liquid gains even more energy the molecules gain enough energy to move completely apart and
evaporate. Ask students to break all bonds with each other and move around the room, spreading out
to fill the room. Students can be asked to lose energy and become a liquid again (condensation), then
a solid (freezing).
You might also like
- What's The Matter? Solids, Liquids, and Gases!: Learning ObjectivesDocument2 pagesWhat's The Matter? Solids, Liquids, and Gases!: Learning ObjectivesJean CamayNo ratings yet
- What's The Matter? Solids, Liquids, and Gases!: Learning ObjectivesDocument2 pagesWhat's The Matter? Solids, Liquids, and Gases!: Learning ObjectivesFranklin BayaniNo ratings yet
- Solids Liquids Gases-Lesson PlanDocument2 pagesSolids Liquids Gases-Lesson Planapi-374307299100% (2)
- Department of Education: Malvar School of Arts and Trade GRADE 8 ScienceDocument4 pagesDepartment of Education: Malvar School of Arts and Trade GRADE 8 ScienceKen Mitchell MoralesNo ratings yet
- Jitorres - The Three States of MatterDocument2 pagesJitorres - The Three States of MatterSAMANTHA GABRIELA MESA REYESNo ratings yet
- 305 Lesson 1 States of Matter 4Document8 pages305 Lesson 1 States of Matter 4api-532271660No ratings yet
- LESSON 1: The Particle Nature of MatterDocument10 pagesLESSON 1: The Particle Nature of MatterPeterClomaJr.No ratings yet
- Grade 3 Properties of Matter Unit (Old Curric.)Document69 pagesGrade 3 Properties of Matter Unit (Old Curric.)CJay Arnau0% (1)
- Matter 6 1Document22 pagesMatter 6 1GARIMANo ratings yet
- Csec Chemistry Notes 1Document2 pagesCsec Chemistry Notes 1debestie100% (3)
- Properties of Matter Lesson PlanDocument2 pagesProperties of Matter Lesson Planapi-251319755100% (1)
- Science Matter Lesson PlanDocument2 pagesScience Matter Lesson Planapi-218287701100% (1)
- States of MatterDocument3 pagesStates of MatterMOHAMMAD PERWEZ ALAM MOHAMMAD AFTAB ALAMNo ratings yet
- Grade 5 Properties of Matter WorksheetsDocument3 pagesGrade 5 Properties of Matter WorksheetsCassandra50% (4)
- Lesson 1Document2 pagesLesson 1linda smithNo ratings yet
- Conformed SCI-8 Quarter3 Module 1Document11 pagesConformed SCI-8 Quarter3 Module 1Thea Marie VilladolidNo ratings yet
- Annotated Unit Plan ObrienDocument4 pagesAnnotated Unit Plan Obrienapi-217292555No ratings yet
- Solid Liquid Gas (3rd Grade Science)Document2 pagesSolid Liquid Gas (3rd Grade Science)OfficialVoki100% (5)
- States of Matter Solids, Liquids, and Gases: Duration: Grade: Number of Students: ObjectivesDocument3 pagesStates of Matter Solids, Liquids, and Gases: Duration: Grade: Number of Students: ObjectivesHiba AmmarNo ratings yet
- Thematic Lesson PlanDocument9 pagesThematic Lesson Planapi-503851228100% (1)
- Middle School ChemistryDocument691 pagesMiddle School ChemistryChristian Homeschool-HubNo ratings yet
- Portfolio Standard 1 - Matter Unit Day 3 LessonDocument4 pagesPortfolio Standard 1 - Matter Unit Day 3 Lessonapi-313166146No ratings yet
- Lesson 1 Unit MatterDocument5 pagesLesson 1 Unit Matterapi-352339425No ratings yet
- EDT 415 Lesson PlanDocument6 pagesEDT 415 Lesson PlanAnonymous POu2y6No ratings yet
- States of Matter ActivitiesDocument5 pagesStates of Matter ActivitiesPoy AlisNo ratings yet
- g5 l1.1 Matter Is Made of ParticlesDocument7 pagesg5 l1.1 Matter Is Made of ParticlessbabuNo ratings yet
- Universal Design For Learning Lesson - States of MatterDocument10 pagesUniversal Design For Learning Lesson - States of Matterapi-337402661No ratings yet
- Stem 534 Lesson PlanDocument7 pagesStem 534 Lesson Planapi-710456380No ratings yet
- ADGE2 Worksheet 1Document2 pagesADGE2 Worksheet 1Jayson ValdezNo ratings yet
- Density Lesson Plan: Science Standards AddressedDocument3 pagesDensity Lesson Plan: Science Standards AddressedKiray EscarezNo ratings yet
- Learning Package in ScienceDocument15 pagesLearning Package in ScienceAbi Comia100% (1)
- Appendix F: Georgia Southwestern State University Lesson PlanDocument7 pagesAppendix F: Georgia Southwestern State University Lesson Planapi-357488871No ratings yet
- National Science Teachers AssociationDocument2 pagesNational Science Teachers AssociationPolitio MonsaludNo ratings yet
- Subject-Physics CHAPTER-1 (Matter) Class - 8Document8 pagesSubject-Physics CHAPTER-1 (Matter) Class - 8kamal_satnaNo ratings yet
- Lesson Plan Revison LLTDocument4 pagesLesson Plan Revison LLTapi-237660366No ratings yet
- Semi-Detailed Lesson Plan in Science 8Document2 pagesSemi-Detailed Lesson Plan in Science 8Vergenia EspielNo ratings yet
- October 2nd 2023-9L-PARTICLE THEORY AND PARTICLE ARRANGEMENTDocument9 pagesOctober 2nd 2023-9L-PARTICLE THEORY AND PARTICLE ARRANGEMENTjhoyvanNo ratings yet
- QUARTER 3 SCIENCE 8 LESSON 1 Properties of The Three States of MatterDocument27 pagesQUARTER 3 SCIENCE 8 LESSON 1 Properties of The Three States of MatterJose BundalianNo ratings yet
- Science 8: Quarter 3 Week 1 and 2Document12 pagesScience 8: Quarter 3 Week 1 and 2Baja, Ysabelle Rose B.No ratings yet
- States of MatterDocument4 pagesStates of MatterabubakaarbuttNo ratings yet
- 6th Grade Science LPDocument3 pages6th Grade Science LPapi-372799212No ratings yet
- Educ424 Curriculum UnitDocument9 pagesEduc424 Curriculum Unitapi-199099982No ratings yet
- Middle School ChemistryDocument691 pagesMiddle School ChemistryGera JankaNo ratings yet
- S2 A Chemistry New Class JuneDocument25 pagesS2 A Chemistry New Class Junethiri hsuNo ratings yet
- SLG UnitDocument44 pagesSLG Unitapi-319172800No ratings yet
- Chemistry Grade - 11 Note - Unit - 3 (2016 E .C)Document11 pagesChemistry Grade - 11 Note - Unit - 3 (2016 E .C)bisratNo ratings yet
- Unit Outline Template: Grade Subject: TitleDocument3 pagesUnit Outline Template: Grade Subject: TitleTyrea PriceNo ratings yet
- Activity SequenceDocument3 pagesActivity Sequenceapi-249789820No ratings yet
- Reflective Lesson Plan-ScienceDocument12 pagesReflective Lesson Plan-Scienceapi-242013201No ratings yet
- Lesson Plan of Comparing Properties of SolidsDocument5 pagesLesson Plan of Comparing Properties of Solidsmary joy vertulfoNo ratings yet
- Lesson Plan Adaptation AssignmentDocument3 pagesLesson Plan Adaptation Assignmentapi-445512320No ratings yet
- Janes Solids Liquids Lesson PlanDocument4 pagesJanes Solids Liquids Lesson Planapi-247261610No ratings yet
- Lesson PlansDocument10 pagesLesson Plansasma amjadNo ratings yet
- Lesson PlanDocument8 pagesLesson Planapi-249789820100% (3)
- Lesson Objective Lesson PlanDocument8 pagesLesson Objective Lesson PlanJennielyn de VeraNo ratings yet
- GENERAL CHEMISTRY 2 Q3 SLM4 FinalDocument17 pagesGENERAL CHEMISTRY 2 Q3 SLM4 FinalKairuuuNo ratings yet
- 6pd Physics Note 10Document14 pages6pd Physics Note 10Jr. WakorNo ratings yet
- Three States of Matter - Realyn D. TanecaDocument11 pagesThree States of Matter - Realyn D. TanecaEya Delos Santos TañecaNo ratings yet
- Work and Power CalculationDocument16 pagesWork and Power CalculationMark Cliffton BadlonNo ratings yet
- Checklist of Requirements OMNIBUS NEW FORMATDocument1 pageChecklist of Requirements OMNIBUS NEW FORMATLarven GinerNo ratings yet
- First Week of Classes For Sy:2021-2022: Grade 8-RubyDocument2 pagesFirst Week of Classes For Sy:2021-2022: Grade 8-RubyRenante CruzNo ratings yet
- Actvity ApplicationDocument1 pageActvity ApplicationRenante CruzNo ratings yet
- Art CurDocument22 pagesArt CurRenante CruzNo ratings yet
- Front Page RankingDocument1 pageFront Page RankingRenante CruzNo ratings yet
- Daily Lesson Plan Daily Lesson Plan: Prepared byDocument1 pageDaily Lesson Plan Daily Lesson Plan: Prepared byRenante CruzNo ratings yet
- Week 1 Mapeh 8 ArtDocument3 pagesWeek 1 Mapeh 8 ArtRenante Cruz50% (2)
- The Learners Demonstrate An Understanding Of. The Learners Shall Be Able ToDocument2 pagesThe Learners Demonstrate An Understanding Of. The Learners Shall Be Able ToRenante CruzNo ratings yet
- Tle 7 Week 1Document7 pagesTle 7 Week 1Renante CruzNo ratings yet
- RulesDocument19 pagesRulesRenante CruzNo ratings yet
- Sortng CardDocument2 pagesSortng CardRenante CruzNo ratings yet
- The Learners Demonstrate An Understanding Of. The Learners Shall Be Able ToDocument3 pagesThe Learners Demonstrate An Understanding Of. The Learners Shall Be Able ToRenante CruzNo ratings yet
- 5.1 Compounds and Chemical FormulasDocument35 pages5.1 Compounds and Chemical Formulastrisha pacleb100% (1)
- Activity #1 AGENDocument4 pagesActivity #1 AGENRenante CruzNo ratings yet
- Detailed Lesson Plan in Science 7Document3 pagesDetailed Lesson Plan in Science 7LeizylAlcantaraNo ratings yet
- Semi-Detailed Lesson Plan. Renante S. Cruz Science Teacher ApplicantDocument5 pagesSemi-Detailed Lesson Plan. Renante S. Cruz Science Teacher ApplicantRenante Cruz100% (1)
- Learning Competencies: Explain Basic Concepts in CookeryDocument8 pagesLearning Competencies: Explain Basic Concepts in CookeryRenante CruzNo ratings yet
- Learning Competencies: Explain Basic Concepts in CookeryDocument8 pagesLearning Competencies: Explain Basic Concepts in CookeryRenante CruzNo ratings yet
- Science 8 Week 1Document67 pagesScience 8 Week 1Renante CruzNo ratings yet
- Aralin OaDocument1 pageAralin OaRenante CruzNo ratings yet
- Whats in The Box Challenhe Motivational Opening in GroupsDocument1 pageWhats in The Box Challenhe Motivational Opening in GroupsRenante CruzNo ratings yet
- Agrarian ReformDocument46 pagesAgrarian ReformRenante Cruz100% (2)
- Demonstrates Understanding Of: Online Resource For Middle School Chemistry. Retrieved FromDocument7 pagesDemonstrates Understanding Of: Online Resource For Middle School Chemistry. Retrieved FromRenante CruzNo ratings yet
- 45Document1 page45Renante CruzNo ratings yet
- Renante Demo 4rank8uinDocument5 pagesRenante Demo 4rank8uinRenante CruzNo ratings yet
- DiagramDocument2 pagesDiagramRenante CruzNo ratings yet
- Republic of The PhilippinesDocument2 pagesRepublic of The PhilippinesRenante CruzNo ratings yet
- Application Letter San MiueDocument1 pageApplication Letter San MiueRenante CruzNo ratings yet
- Letter of ConsentDocument1 pageLetter of ConsentRenante Cruz100% (2)
- STEM Labs for Physical Science, Grades 6 - 8From EverandSTEM Labs for Physical Science, Grades 6 - 8Rating: 3.5 out of 5 stars3.5/5 (6)
- How to Teach Nature Journaling: Curiosity, Wonder, AttentionFrom EverandHow to Teach Nature Journaling: Curiosity, Wonder, AttentionRating: 4.5 out of 5 stars4.5/5 (3)
- A-level Biology Revision: Cheeky Revision ShortcutsFrom EverandA-level Biology Revision: Cheeky Revision ShortcutsRating: 5 out of 5 stars5/5 (5)
- Lower Secondary Science Workbook: Stage 8From EverandLower Secondary Science Workbook: Stage 8Rating: 5 out of 5 stars5/5 (1)
- Nature-Based Learning for Young Children: Anytime, Anywhere, on Any BudgetFrom EverandNature-Based Learning for Young Children: Anytime, Anywhere, on Any BudgetRating: 5 out of 5 stars5/5 (1)
- Nature Play Workshop for Families: A Guide to 40+ Outdoor Learning Experiences in All SeasonsFrom EverandNature Play Workshop for Families: A Guide to 40+ Outdoor Learning Experiences in All SeasonsRating: 4 out of 5 stars4/5 (4)
- Interactive Science Notebook: The Human Body WorkbookFrom EverandInteractive Science Notebook: The Human Body WorkbookRating: 4 out of 5 stars4/5 (2)
- Quantum Physics for Beginners: Simple Illustrated Guide to Discover with Practical Explanations the Paradoxes of the Life and Universe Reconsidering RealityFrom EverandQuantum Physics for Beginners: Simple Illustrated Guide to Discover with Practical Explanations the Paradoxes of the Life and Universe Reconsidering RealityRating: 2 out of 5 stars2/5 (1)
- How Do Cell Phones Work? Technology Book for Kids | Children's How Things Work BooksFrom EverandHow Do Cell Phones Work? Technology Book for Kids | Children's How Things Work BooksNo ratings yet
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookNo ratings yet
- Interactive Notebook: Life Science, Grades 5 - 8From EverandInteractive Notebook: Life Science, Grades 5 - 8Rating: 5 out of 5 stars5/5 (4)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- A-Level Chemistry Revision: Cheeky Revision ShortcutsFrom EverandA-Level Chemistry Revision: Cheeky Revision ShortcutsRating: 4 out of 5 stars4/5 (5)
- Nature Preschools and Forest Kindergartens: The Handbook for Outdoor LearningFrom EverandNature Preschools and Forest Kindergartens: The Handbook for Outdoor LearningRating: 3.5 out of 5 stars3.5/5 (3)
- Airplane Flying Handbook: FAA-H-8083-3C (2024)From EverandAirplane Flying Handbook: FAA-H-8083-3C (2024)Rating: 4 out of 5 stars4/5 (12)
- An Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksFrom EverandAn Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksRating: 5 out of 5 stars5/5 (1)