Professional Documents
Culture Documents
Mass and Energy Balance: EB Converted Styrene+hydrogen EB Mixed Fed Reactor
Uploaded by
Porkkodi SugumaranOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mass and Energy Balance: EB Converted Styrene+hydrogen EB Mixed Fed Reactor
Uploaded by
Porkkodi SugumaranCopyright:
Available Formats
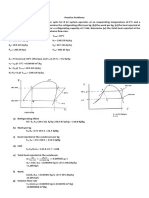
Mass and energy balance
Dehydrogenation of ethylenebenzene to styrene
C6H5CH2CH3 C6H5CH=CH2 + H2 [1] ∆H𝑟 ° (600°C) = + 124.5 kJ/mol
Stoichiometry balance
C6H5CH2CH3 C6H5CH=CH2 H2
(ethylenebenzene) (styrene) (hydrogen)
Coefficient 1 mol 1 mol 1 mol
Molar mass (kg) 106.165 104.15 2
Weight (kg) 158.00 155.00 (product required) 3.00
i) The major reaction [1] is the irreversible endothermic conversion of
ethylenebenzene to styrene and hydrogen. This reaction proceeds catalytically with
high yield.
One pass conversion of ethylenebenzene (EB)
% conversion =
EB converted ¿ styrene+ hydrogen ¿ reactor ¿ x
EB mixed fed ¿
(155+3)
0.35 = x100
EBmixed
EB mixed = 451.43 kg/hr or 4252.15 mol/hr
Styrene produced = 155kg/hr or 1488.24 mol/hr
Hydrogen gas produced = 3kg/hr or 1500 mol/hr
Flow rate of the recycled ethylenebenzene
Unconverted recycle EB = 451.43 kg/hr – (155 kg/hr + 3 kg/hr)
= 293.43 kg/hr or 2764.30 mol/hr
Fresh ethylenebenzene feed rate
Fresh ethylenebenzene = 158 kg/hr or 1488.50 mol/hr (from the stoichiometry table)
Circulation rate of the water
Circulation rate of water depends on the amount of steam required to supply heat to
endothermic reactor. Heat of reaction, ∆H𝑟 ° (600°C) = 124.5 kJ/mol of
ethylenebenzene.
Q = ṁ ∆H𝑟° (600°C)
= 4252.15 x 124.5
= 529392.68 kJ/hr
Steam required to supply heat
Q = ṁ λ water @ 600°C
529392.68 = ṁ (2100 kJ/kg)
ṁ = 252.09 kg/hr
Amount of water circulated = amount of steam required
= 252.09 kg/hr or 14005.00 mol/hr
ii) Required rate of heat input for preheater (A), steam generator (F) and reactor (C)
Ethylenebenzene preheater (A) required heat input
Q = mCpliquid ∆t
= 4252.15 x 182 x (500 – 25)
= 367598367.5 J/hr or 367598.37 kJ/hr
Steam generator (F) required heat input
Q = mCpwater ∆t
= 252.09 x 4.187 x (700 – 25)
= 712463.06 kJ/hr
Reactor input (C) required heat input
As calculated in the circulation rate of water part, heat input (Q)
Q = 529392.68 kJ/hr
You might also like
- Production of 20,000 LPD Biodiesel From Chicken Fat Waste: Project SupervisorDocument36 pagesProduction of 20,000 LPD Biodiesel From Chicken Fat Waste: Project Supervisorsaqib sulmanNo ratings yet
- Hydrogen Energy 2520balance.Document6 pagesHydrogen Energy 2520balance.sahilchemNo ratings yet
- Q - Kelompok 4 - Tugas 3 PUBDocument2 pagesQ - Kelompok 4 - Tugas 3 PUBRediana PutriNo ratings yet
- Reactor:: Energy BalanceDocument4 pagesReactor:: Energy BalanceSanjay KumarNo ratings yet
- Energy BalanceDocument10 pagesEnergy BalanceAadiNo ratings yet
- Mass BalanceDocument20 pagesMass BalanceBhaskar BethiNo ratings yet
- AAS Energy 2520 BalanceDocument3 pagesAAS Energy 2520 Balanceapi-3714811No ratings yet
- Conduction ProblemsDocument2 pagesConduction Problemsmendoza21203831mNo ratings yet
- Performance OF Boilers: Bibin ChidambaranathanDocument58 pagesPerformance OF Boilers: Bibin ChidambaranathanDr. BIBIN CHIDAMBARANATHAN100% (1)
- 19bch023 - 19bch002 (PC Term Paper)Document23 pages19bch023 - 19bch002 (PC Term Paper)Aditya JaniNo ratings yet
- Acetaldehyde Energy 2520 BalanceDocument10 pagesAcetaldehyde Energy 2520 Balanceapi-3714811100% (1)
- DocDocument5 pagesDoccessareNo ratings yet
- Chemical Fuel & Solar CellDocument53 pagesChemical Fuel & Solar CellSachin NaikNo ratings yet
- Nchu-Che Thermodynamics I: This Question Paper Consists of 3 QuestionsDocument3 pagesNchu-Che Thermodynamics I: This Question Paper Consists of 3 QuestionsRohit SharmaNo ratings yet
- Steam Heating ProcessDocument4 pagesSteam Heating ProcessAnonymous VA3KeEwzNo ratings yet
- Heat TransferDocument57 pagesHeat TransferSatyam PandyaNo ratings yet
- Cogeneration Power PlantDocument6 pagesCogeneration Power PlantGanvendra Singh ChaharNo ratings yet
- Solved - Problems in ThermodynamicsDocument29 pagesSolved - Problems in ThermodynamicsAngelica Joyce Benito100% (6)
- C9 Enthalpy PowerpointDocument135 pagesC9 Enthalpy PowerpointHanaa KhaldiNo ratings yet
- Mass&Energy Balance2Document41 pagesMass&Energy Balance2Muhammad Umer RanaNo ratings yet
- Problems On Boilers With SolutionsDocument4 pagesProblems On Boilers With SolutionsSuraj Kumar86% (7)
- Problem Set ODocument19 pagesProblem Set OnimboNo ratings yet
- Problem: Vivek RDocument18 pagesProblem: Vivek RHritik LalNo ratings yet
- Coal Fired Boiler StudyDocument8 pagesCoal Fired Boiler StudyMuzamil ShahidNo ratings yet
- University of The West Indies, St. Augustine.: FacultyDocument8 pagesUniversity of The West Indies, St. Augustine.: FacultyCharlotte BNo ratings yet
- Energy BalanceDocument11 pagesEnergy BalanceBharat VaajNo ratings yet
- CalorimetryDocument20 pagesCalorimetrySB KP100% (1)
- Chapter 12 (Vapor Power Systems) : Actual Rankine CycleDocument8 pagesChapter 12 (Vapor Power Systems) : Actual Rankine CycleNagham MuradNo ratings yet
- Linked Que 4-7Document5 pagesLinked Que 4-7Pranav MishraNo ratings yet
- Energy Balance: Technological Institute of The PhilippinesDocument56 pagesEnergy Balance: Technological Institute of The Philippineshenriel tambioNo ratings yet
- Fuels and Combustion: - Calorific Value - Significance and Comparison Between LCV andDocument46 pagesFuels and Combustion: - Calorific Value - Significance and Comparison Between LCV andSandhya SundarNo ratings yet
- Manufacture of CumeneDocument46 pagesManufacture of CumeneG Vamsee KrishnaNo ratings yet
- Fuels and CombustionDocument48 pagesFuels and CombustionAMAL MATHEWNo ratings yet
- Eto Na Lex 2 10Document10 pagesEto Na Lex 2 10Alexis CarpenaNo ratings yet
- Energy Performance Assessment of Boilers: Boiler Efficiency by Direct MethodDocument19 pagesEnergy Performance Assessment of Boilers: Boiler Efficiency by Direct MethodAlif Nur FirdausNo ratings yet
- Utility Cost Estimate: Associate Professor Dr. Deacha Chatsiriwech Chemical Engineering, Chulalongkorn UniversityDocument16 pagesUtility Cost Estimate: Associate Professor Dr. Deacha Chatsiriwech Chemical Engineering, Chulalongkorn UniversitySitiMursidahNo ratings yet
- Revision - Energy BalanceDocument4 pagesRevision - Energy BalancePorkkodi SugumaranNo ratings yet
- Procss Design and Mass BalanceDocument7 pagesProcss Design and Mass BalanceBa Tawa NaNo ratings yet
- Evap Cond F F G G Sat SatDocument17 pagesEvap Cond F F G G Sat SatAlexis CarpenaNo ratings yet
- TD WorksheetDocument4 pagesTD WorksheetrtyiookNo ratings yet
- Tutorial Problems (Set 7) PDFDocument3 pagesTutorial Problems (Set 7) PDFManishaa Varatha RajuNo ratings yet
- Energy BalanceDocument19 pagesEnergy Balancekamran AhmadNo ratings yet
- CH 06Document15 pagesCH 06hirenpatel_universalNo ratings yet
- Thermochemistry (UCE) - AKHS Edition 2020Document16 pagesThermochemistry (UCE) - AKHS Edition 2020Kim SewoonNo ratings yet
- Fuels and Combustion: - Calorific Value - Significance and Comparison Between LCV andDocument44 pagesFuels and Combustion: - Calorific Value - Significance and Comparison Between LCV andanurag prernaNo ratings yet
- Thermodynamics 7 Steam TurbineDocument5 pagesThermodynamics 7 Steam Turbinep_nicks89No ratings yet
- Chapter 3 (B) Energy Balance: 3.9 AssumptionsDocument21 pagesChapter 3 (B) Energy Balance: 3.9 Assumptionssaur1No ratings yet
- ME413 (Cogeneration) (261117) 1Document8 pagesME413 (Cogeneration) (261117) 1md mahdiNo ratings yet
- Mod 6 Fuels and CombustionDocument58 pagesMod 6 Fuels and CombustionVarsha VarmaNo ratings yet
- Chlorine Energy 2520 BalanceDocument4 pagesChlorine Energy 2520 Balancetonzz10No ratings yet
- Calorific Value Lecture 3, Fuel Tech-LlDocument12 pagesCalorific Value Lecture 3, Fuel Tech-LlShakeel AhmadNo ratings yet
- Combustion Notes (University Level)Document44 pagesCombustion Notes (University Level)Devdutt Sharma100% (1)
- Steam Cycle Lecture 1 RankineDocument5 pagesSteam Cycle Lecture 1 RankineFerdinand AlcantaraNo ratings yet
- Tutorial 2Document5 pagesTutorial 2Debashish KalitaNo ratings yet
- Power Plant New QuestionDocument2 pagesPower Plant New QuestionRishav niroulaNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Synthetic Natural Gas: From Coal, Dry Biomass, and Power-to-Gas ApplicationsFrom EverandSynthetic Natural Gas: From Coal, Dry Biomass, and Power-to-Gas ApplicationsTilman J. SchildhauerNo ratings yet
- YearDocument2 pagesYearPorkkodi SugumaranNo ratings yet
- Polish Journal of Chemical Technology - 4 - 2007 - LubkowskiDocument4 pagesPolish Journal of Chemical Technology - 4 - 2007 - LubkowskiPorkkodi SugumaranNo ratings yet
- PRJ61203: Chapter 1: Evaluation of Previous Group Project (5%)Document7 pagesPRJ61203: Chapter 1: Evaluation of Previous Group Project (5%)Porkkodi SugumaranNo ratings yet
- 1 s2.0 S1878535220302501 MainDocument9 pages1 s2.0 S1878535220302501 MainPorkkodi SugumaranNo ratings yet
- ArjunB TechProjectHeatExchangerCFD PDFDocument45 pagesArjunB TechProjectHeatExchangerCFD PDFHusnainNo ratings yet
- 1 s2.0 S1878535220302501 MainDocument9 pages1 s2.0 S1878535220302501 MainPorkkodi SugumaranNo ratings yet
- Controlled Release FertilizersDocument5 pagesControlled Release FertilizersPorkkodi SugumaranNo ratings yet
- 1 Temperature Control Lab v1 PDFDocument1 page1 Temperature Control Lab v1 PDFPorkkodi SugumaranNo ratings yet
- AssignmentDocument5 pagesAssignmentPorkkodi SugumaranNo ratings yet
- Temperature Control Lab CHBE 356: Group KDocument13 pagesTemperature Control Lab CHBE 356: Group KPorkkodi SugumaranNo ratings yet
- 1 Temperature Control Lab v1 PDFDocument1 page1 Temperature Control Lab v1 PDFPorkkodi SugumaranNo ratings yet
- AssignmentDocument5 pagesAssignmentPorkkodi SugumaranNo ratings yet
- Table? Shows Properties of MethanolDocument11 pagesTable? Shows Properties of MethanolPorkkodi SugumaranNo ratings yet
- Pet (AutoRecovered)Document10 pagesPet (AutoRecovered)Porkkodi SugumaranNo ratings yet
- Part C: O (Carbon H O (Propane) - The Formation of Hydrates Could Be in The Presence ofDocument4 pagesPart C: O (Carbon H O (Propane) - The Formation of Hydrates Could Be in The Presence ofPorkkodi SugumaranNo ratings yet
- DocumentDocument1 pageDocumentPorkkodi SugumaranNo ratings yet
- Piping SystemDocument1 pagePiping SystemPorkkodi SugumaranNo ratings yet
- Week 1 - Introduction - March 2021Document6 pagesWeek 1 - Introduction - March 2021Porkkodi SugumaranNo ratings yet
- Vessel support system: weight of the shell=π r r pgDocument3 pagesVessel support system: weight of the shell=π r r pgPorkkodi SugumaranNo ratings yet
- PRJ61203: Chapter 1: Evaluation of Previous Group Project (5%)Document7 pagesPRJ61203: Chapter 1: Evaluation of Previous Group Project (5%)Porkkodi SugumaranNo ratings yet
- Section BDocument1 pageSection BPorkkodi SugumaranNo ratings yet
- Week 11 Mechanical DesignDocument76 pagesWeek 11 Mechanical DesignPorkkodi SugumaranNo ratings yet
- Applied Energy: Laura A. Pellegrini, Giorgio Soave, Simone Gamba, Stefano LangèDocument7 pagesApplied Energy: Laura A. Pellegrini, Giorgio Soave, Simone Gamba, Stefano LangèPorkkodi SugumaranNo ratings yet
- ppd9 PDFDocument7 pagesppd9 PDFPorkkodi SugumaranNo ratings yet
- Simulation of Biomethanol Production From Green Syngas Through Sustainable Process DesignDocument9 pagesSimulation of Biomethanol Production From Green Syngas Through Sustainable Process DesignPorkkodi SugumaranNo ratings yet
- PPD 5Document113 pagesPPD 5Porkkodi SugumaranNo ratings yet
- Design and Analysis of PID Controller For CSTR Process: Nikul Maheshwari, Neha Jain, Arun Jingar, Mahendra SutharDocument4 pagesDesign and Analysis of PID Controller For CSTR Process: Nikul Maheshwari, Neha Jain, Arun Jingar, Mahendra SutharPorkkodi SugumaranNo ratings yet
- ChemicalEngineering DrugProduct Ch23 NielsNicolai PhDThesis PDFDocument34 pagesChemicalEngineering DrugProduct Ch23 NielsNicolai PhDThesis PDFPorkkodi SugumaranNo ratings yet
- We Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsDocument37 pagesWe Are Intechopen, The World'S Leading Publisher of Open Access Books Built by Scientists, For ScientistsPorkkodi SugumaranNo ratings yet
- Jurnal: Daur LingkunganDocument4 pagesJurnal: Daur LingkunganAli UsmanNo ratings yet
- WavesDocument7 pagesWavesRekha PrasadNo ratings yet
- The Serpentine Mineral GroupDocument3 pagesThe Serpentine Mineral GroupYudhi PrawiraNo ratings yet
- Inorganic ChemistryDocument2 pagesInorganic ChemistryTaqeeb AbbasNo ratings yet
- The Ecology of The CityDocument4 pagesThe Ecology of The CityCARLOS SABOGALNo ratings yet
- Electricity Generation From Citrofortunella Microcarpa Calamansi and Musa Balbisiana Saba Banana Using Dual Chamber Microbial Fuel CellDocument25 pagesElectricity Generation From Citrofortunella Microcarpa Calamansi and Musa Balbisiana Saba Banana Using Dual Chamber Microbial Fuel CellHarrie Floyd C. LelisNo ratings yet
- Hotel Engineering & MaintenanceDocument2 pagesHotel Engineering & MaintenanceKamlesh ChouhanNo ratings yet
- Cervac Board HS&HS PlusDocument2 pagesCervac Board HS&HS PlusCynthia MillerNo ratings yet
- Indian PatentDocument6 pagesIndian PatentCHANDANNo ratings yet
- Concept Summary: Batesville High School PhysicsDocument20 pagesConcept Summary: Batesville High School PhysicssbdmanNo ratings yet
- Inside Our Earth Class 7 MCQs Questions With AnswersDocument5 pagesInside Our Earth Class 7 MCQs Questions With AnswersKalai Selvi MohanNo ratings yet
- Power System Protection LabDocument25 pagesPower System Protection LabShowkat Hossen SabujNo ratings yet
- African Knowledges and SciencesDocument176 pagesAfrican Knowledges and SciencesSNNo ratings yet
- Added Photos-Final PROJECT REPOR 8sem (1) PDFDocument50 pagesAdded Photos-Final PROJECT REPOR 8sem (1) PDFravi singhNo ratings yet
- Design of Ground Source HeatpumpsDocument119 pagesDesign of Ground Source Heatpumpsmnt6176No ratings yet
- Kids Fight Climate Change: Act Now To Be A #2minutesuperhero Chapter SamplerDocument26 pagesKids Fight Climate Change: Act Now To Be A #2minutesuperhero Chapter SamplerCandlewick Press100% (1)
- Sales Proposal 291KW Karachi Club Annex v1Document7 pagesSales Proposal 291KW Karachi Club Annex v1junaid ahmadNo ratings yet
- Exercise #1 - Physical PropertiesDocument3 pagesExercise #1 - Physical PropertiesVieno Gino CruzNo ratings yet
- 2017 - 10 - 05 - Propane Isotherms On The PV Plane Using The Peng-Robinson Equation of StateDocument13 pages2017 - 10 - 05 - Propane Isotherms On The PV Plane Using The Peng-Robinson Equation of StateshalalalaNo ratings yet
- To Find The Moisture Content in A SoilDocument4 pagesTo Find The Moisture Content in A Soilliaqt zaib khanNo ratings yet
- Lecture 19w Groundwater 1 Darcy PowerpointDocument19 pagesLecture 19w Groundwater 1 Darcy PowerpointAmir ShahzadNo ratings yet
- HaRa Solutions Brochure NewDocument2 pagesHaRa Solutions Brochure NewvanilavarasuNo ratings yet
- Technical Standard For SolarDocument44 pagesTechnical Standard For SolarAbhinav SinhaNo ratings yet
- DLP For Co1 Science 4 - BagokristelfinalDocument7 pagesDLP For Co1 Science 4 - BagokristelfinalAlex Jr. FeranilNo ratings yet
- Petroleum SystemsDocument20 pagesPetroleum Systemsmohamed elshemyNo ratings yet
- Physical Sciences Ieb NSC Grade 12 Past Exam Papers 2016 p1 Data SheetDocument2 pagesPhysical Sciences Ieb NSC Grade 12 Past Exam Papers 2016 p1 Data Sheetoluhlevumisa07No ratings yet
- Cartographie de L'érosion Hydrique Des Sols Et Priorisation Des Mesures de Conservation Dans Le Territoire D'uvira (République Démocratique Du Congo)Document35 pagesCartographie de L'érosion Hydrique Des Sols Et Priorisation Des Mesures de Conservation Dans Le Territoire D'uvira (République Démocratique Du Congo)CasedepartNo ratings yet
- Recycling On PlasticsDocument15 pagesRecycling On PlasticskunalNo ratings yet
- Application of GIS in Disaster ManagementDocument10 pagesApplication of GIS in Disaster ManagementPRAVIN ANNAMALAINo ratings yet
- Handbook For Energy Storage SystemsDocument36 pagesHandbook For Energy Storage SystemsNguyễn Thanh TùngNo ratings yet