Professional Documents
Culture Documents
Jee Chemistry - 5
Uploaded by
Avinash BillaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Jee Chemistry - 5
Uploaded by
Avinash BillaCopyright:
Available Formats
ARCHIVE - JEE (Main) CHEMISTRY
27. The ratio of the shortest wavelength of two spectral 4a0 4a0
series of hydrogen spectrum is found to be about (1) (2)
3 9
9. The spectral series are [JEE (Main)-2019] 2a0 2a0
(3) (4)
(1) Paschen and Pfund (2) Brackett and Pfund 3 9
(3) Lyman and Paschen (4) Balmer and Brackett 33. The de Broglie wavelength of an electron in the 4th
Bohr orbit is [JEE (Main)-2020]
28. The electrons are more likely to be found
(1) 4a0 (2) 6a0
[JEE (Main)-2019] (3) 8a0 (4) 2a0
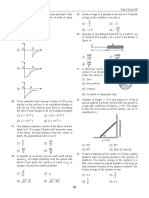
34. The figure that is not a direct manifestation of the
a (x) quantum nature of atoms is [JEE (Main)-2020]
Increasing wavelength
b x
–x
(1)
c
Absorption spectrum
(1) In the region a and c (2) Only in the region c
Internal
(3) Only in the region a (4) In the region a and b energy
(2) of
29. Among the following, the energy of 2s orbital is Ar 300 400 500 600
lowest in [JEE (Main)-2019]
Temperature (K)
(1) Li (2) K
(3) H (4) Na T2 > T1
Intensity
30. The number of orbitals associated with quantum of black body
(3) radiation T1
1
numbers n = 5, ms = is [JEE (Main)-2020]
2 Wavelength
(1) 15 (2) 50 Rb K
Na
Kinetic
(3) 25 (4) 11 energy of
(4) photoelectrons
31. For the Balmer series in the spectrum of H atom,
Frequency of incident
⎪⎧ 1 1 ⎪⎫ radiation
RH ⎨ 2 2 ⎬ , the correct statements among (I)
⎪⎩ n1 n2 ⎪⎭ 35. The number of subshells associated with n = 4 and
m = –2 quantum numbers is [JEE (Main)-2020]
to (VI) are
(1) 2 (2) 8
(I) As wavelength decreases, the lines in the (3) 4 (4) 16
series converge
36. The region in the electromagnetic spectrum where
(II) The integer n1 is equal to 2 the Balmar series lines appear is
(III) The lines of longest wavelength corresponds to [JEE (Main)-2020]
n2 = 3 (1) Microwave (2) Ultraviolet
(IV) The ionization energy of hydrogen can be (3) Visible (4) Infrared
calculated from wave number of these lines 37. The shortest wavelength of H atom in the Lyman
series is 1. The longest wavelength in the Balmar
[JEE (Main)-2020]
series of He+ is [JEE (Main)-2020]
(1) (I), (II), (III) (2) (II), (III), (IV)
51 361
(3) (I), (III), (IV) (4) (I), (II), (IV) (1) (2)
9 5
32. The radius of the second Bohr orbit, in terms of the 271 91
Bohr radius, a0, in Li2+ is [JEE (Main)-2020] (3) (4)
5 5
You might also like
- Power Plant Chemistry by Ramesh PDFDocument62 pagesPower Plant Chemistry by Ramesh PDFKomma Ramesh0% (1)
- Gas Laws (Notes) PDFDocument9 pagesGas Laws (Notes) PDFHassan Jamal100% (1)
- Chemistry Atomic-StructureDocument10 pagesChemistry Atomic-Structuremangeshchavan980No ratings yet
- P Ch-25 AtomDocument6 pagesP Ch-25 AtomKartik 1081No ratings yet
- Jee Chemistry - 4Document1 pageJee Chemistry - 4Avinash BillaNo ratings yet
- Atoms - Modern PhysicsDocument13 pagesAtoms - Modern PhysicsborntwofukNo ratings yet
- 649bc82b89417e0018f4647a - ## - Atomic Structure - Practice Sheet - Arjuna NEET 2024Document2 pages649bc82b89417e0018f4647a - ## - Atomic Structure - Practice Sheet - Arjuna NEET 2024Lalit SinghNo ratings yet
- Atomic Structure NewDocument2 pagesAtomic Structure NewAditya RamNo ratings yet
- Atomic Structure Jee Main SelectedDocument5 pagesAtomic Structure Jee Main SelectedfopjfvmhdNo ratings yet
- USEFUL DATA: σ = 5.6697 a bx b x dx bx: e a r aDocument2 pagesUSEFUL DATA: σ = 5.6697 a bx b x dx bx: e a r aHarsh TiwariNo ratings yet
- Home Assignment-1Document13 pagesHome Assignment-1ansh guptaNo ratings yet
- Concept Strengthening Sheet CSS-01 Chemistry: Q.82 (Code-A) (Wave and Particle Nature of Light)Document6 pagesConcept Strengthening Sheet CSS-01 Chemistry: Q.82 (Code-A) (Wave and Particle Nature of Light)sheheryarNo ratings yet
- 3001 Physics Paper With Answer MorningDocument6 pages3001 Physics Paper With Answer Morningspicypip123No ratings yet
- Electromagnetic WavesDocument5 pagesElectromagnetic Wavesfstmission123No ratings yet
- Aiatsoymeo2016t06 SolutionDocument29 pagesAiatsoymeo2016t06 Solutionsanthosh7kumar-24No ratings yet
- Atomic Structure-DTS-2 Main (Archive)Document3 pagesAtomic Structure-DTS-2 Main (Archive)Halfborn GundersonNo ratings yet
- Test 15 - PaperDocument17 pagesTest 15 - PaperAashika DhareNo ratings yet
- Adobe Scan Aug 19, 2022Document5 pagesAdobe Scan Aug 19, 2022Kunal GoutamNo ratings yet
- Electromagnetic WavesDocument5 pagesElectromagnetic WavesSarthak ShingareNo ratings yet
- Gate 2018 PHDocument7 pagesGate 2018 PHPasupuleti AnilNo ratings yet
- @PW - Yakeen - Batchatoms Lec 02 DPPDocument3 pages@PW - Yakeen - Batchatoms Lec 02 DPPAnand RockyNo ratings yet
- T Est - 6: (Physics)Document30 pagesT Est - 6: (Physics)santhosh7kumar-24No ratings yet
- Atoms - DPP 01 (Of Lecture 03) - Lakshya NEET Fastrack 2024Document4 pagesAtoms - DPP 01 (Of Lecture 03) - Lakshya NEET Fastrack 2024patelayushactionNo ratings yet
- Electric Charge and Field - DPP 08 - Pragati (PCM) KannadaDocument3 pagesElectric Charge and Field - DPP 08 - Pragati (PCM) Kannadamanojmanu113manuNo ratings yet
- Phy-Iv-04 (R) PDFDocument4 pagesPhy-Iv-04 (R) PDFlp eelceeNo ratings yet
- Moving Charge & Magnetism - DPP 06 (Of Lec 08)Document3 pagesMoving Charge & Magnetism - DPP 06 (Of Lec 08)rambhaiya888No ratings yet
- 02 Poll - C - 02 (Chemistry) Question PartDocument3 pages02 Poll - C - 02 (Chemistry) Question PartMag GamingNo ratings yet
- ATOMS - Practice Sheet & Solution - Vijeta 2023Document5 pagesATOMS - Practice Sheet & Solution - Vijeta 2023siyaNo ratings yet
- Atomic Structure: ChemistryDocument8 pagesAtomic Structure: ChemistryGowtham BurleNo ratings yet
- ATOMIC STRUCTURE NEET Previous Year Q Bank Till 2020Document9 pagesATOMIC STRUCTURE NEET Previous Year Q Bank Till 2020Arnav SinghalNo ratings yet
- Short Practice Test 03 - Physics - Lakshya JEE 2.0 2024Document1 pageShort Practice Test 03 - Physics - Lakshya JEE 2.0 2024Deep SrivastavaNo ratings yet
- P Ch-23 AtomsDocument5 pagesP Ch-23 Atomsmysoftinfo.incNo ratings yet
- Atomic Structure PDFDocument14 pagesAtomic Structure PDFbunny reedNo ratings yet
- Atoms PYQDocument8 pagesAtoms PYQAyaanNo ratings yet
- Atomic Structure - Practice Sheet - Lakshya 11th JEE Rapid Revision CourseDocument8 pagesAtomic Structure - Practice Sheet - Lakshya 11th JEE Rapid Revision CourseAnvi jainNo ratings yet
- Useful Data: C 3 A: e A R ADocument2 pagesUseful Data: C 3 A: e A R AHarsh TiwariNo ratings yet
- Exercise - 1 - 1649679708 (1) SjjsDocument26 pagesExercise - 1 - 1649679708 (1) SjjsShubhamNo ratings yet
- Aakash Rank Booster Test Series For NEET-2020Document16 pagesAakash Rank Booster Test Series For NEET-2020Anish TakshakNo ratings yet
- Allen: Final Jee-Main Examination - March, 2021Document5 pagesAllen: Final Jee-Main Examination - March, 2021Again MishraNo ratings yet
- 3101 Physics Paper With Answer MorningDocument5 pages3101 Physics Paper With Answer Morningmonishbro41No ratings yet
- Jee Chemistry - 3Document1 pageJee Chemistry - 3Avinash BillaNo ratings yet
- Neet Paper 2021: Section - A (Physics) AnsDocument21 pagesNeet Paper 2021: Section - A (Physics) AnsMission NEET 2022No ratings yet
- Structure of Atom PYQsDocument15 pagesStructure of Atom PYQsPrince mandalNo ratings yet
- Chemistry Full Set-September-FullsetDocument40 pagesChemistry Full Set-September-FullsetAbhiNo ratings yet
- Atoms and NucleiDocument16 pagesAtoms and NucleiNINE EDUCATIONNo ratings yet
- Modern Physics 1Document18 pagesModern Physics 1Harsh GuptaNo ratings yet
- AIATS-02 (OYM) @DefeatNEETDocument21 pagesAIATS-02 (OYM) @DefeatNEETAadesh SharmaNo ratings yet
- 2IIT1920 (IIT Camp) (Main) CWS01 (Atomic Structure, Periodic Properties and Chemical Bonding) (SAG Mam) PDFDocument3 pages2IIT1920 (IIT Camp) (Main) CWS01 (Atomic Structure, Periodic Properties and Chemical Bonding) (SAG Mam) PDFvidhit dlNo ratings yet
- The Previous Year NEET Question Paper With The Answer Key For The Year 2023 Only On Zephyr.Document43 pagesThe Previous Year NEET Question Paper With The Answer Key For The Year 2023 Only On Zephyr.Zephyr EntranceNo ratings yet
- Physics Q4Document7 pagesPhysics Q4Rachita RamanNo ratings yet
- Practice Test 02 - ChemistryDocument2 pagesPractice Test 02 - Chemistrynachivednehete9977No ratings yet
- Mock Test Paper 1-10-2020Document160 pagesMock Test Paper 1-10-2020Parnava Pratihar V BINOY 64No ratings yet
- JEE Main PYQs Atomic Structure 28399275Document22 pagesJEE Main PYQs Atomic Structure 28399275Bharat MevadaNo ratings yet
- Atomic Structure 87Document16 pagesAtomic Structure 87Sarita KhatriNo ratings yet
- Full Syllabus Test Papter No. 2 - Rishabh Sir - AnilDocument19 pagesFull Syllabus Test Papter No. 2 - Rishabh Sir - Anildigvijay singhNo ratings yet
- T.Me/Agexpr EMIDocument11 pagesT.Me/Agexpr EMIHarsh GuptaNo ratings yet
- RM-1 CPP 22 Physics Chemistry Botany 2021Document18 pagesRM-1 CPP 22 Physics Chemistry Botany 2021soyel afridiNo ratings yet
- Structure of AtomDocument6 pagesStructure of AtomPoorni RenuNo ratings yet
- Test - 26: Final Test Series (Online) For JEE (Main) - 2021Document9 pagesTest - 26: Final Test Series (Online) For JEE (Main) - 2021Vishal kumar MauryaNo ratings yet
- Akash Test No 3Document5 pagesAkash Test No 3Prashanth 070No ratings yet
- X-ray Absorption Spectroscopy for the Chemical and Materials SciencesFrom EverandX-ray Absorption Spectroscopy for the Chemical and Materials SciencesNo ratings yet
- Iit JeeDocument4 pagesIit JeeAvinash BillaNo ratings yet
- Phpi 4 SwraDocument19 pagesPhpi 4 SwrainfoNo ratings yet
- Iit JeeDocument4 pagesIit JeeAvinash BillaNo ratings yet
- Formatting An Academic Paper: Document MarginsDocument2 pagesFormatting An Academic Paper: Document MarginsAvinash BillaNo ratings yet
- Jee Chemistry - 3Document1 pageJee Chemistry - 3Avinash BillaNo ratings yet
- Jee Chemistry - 2Document1 pageJee Chemistry - 2Avinash BillaNo ratings yet
- Bangalore FruitsDocument6 pagesBangalore FruitsMahesh GNo ratings yet
- Jee Chemistry - 1Document1 pageJee Chemistry - 1Avinash BillaNo ratings yet
- Class12 Mathematics2 Unit10 NCERT TextBook EnglishEditionDocument39 pagesClass12 Mathematics2 Unit10 NCERT TextBook EnglishEditionsundeepyadav9No ratings yet
- Class12 Mathematics2 Unit10 NCERT TextBook EnglishEditionDocument39 pagesClass12 Mathematics2 Unit10 NCERT TextBook EnglishEditionsundeepyadav9No ratings yet
- ASTM C 128 Standard Test Method For Density, Relative Density (Specific Gravity), and AbsorptionDocument6 pagesASTM C 128 Standard Test Method For Density, Relative Density (Specific Gravity), and AbsorptionRyan LasacaNo ratings yet
- Dokumentips Bridge Engineering by Victor Johnson Essentials of Bridge Engineering VictorpdfDocument2 pagesDokumentips Bridge Engineering by Victor Johnson Essentials of Bridge Engineering VictorpdfVivek KumarNo ratings yet
- Heat Transfer: Mechanical EngineeringDocument10 pagesHeat Transfer: Mechanical EngineeringVenkatasairamreddy KandulaNo ratings yet
- EIM 9 - Q3 - Mod3 - USLeM RTPDocument8 pagesEIM 9 - Q3 - Mod3 - USLeM RTPEduard SantosNo ratings yet
- HPLCDocument2 pagesHPLCApoorva ChaudharyNo ratings yet
- Paul DiracDocument6 pagesPaul DiracLeonNo ratings yet
- Hesss Law WorksheetDocument3 pagesHesss Law WorksheetAtulya BharadwajNo ratings yet
- Chemical+Equilibrium+ +marathon+ (Mohit+Sir) +Document197 pagesChemical+Equilibrium+ +marathon+ (Mohit+Sir) +Sanjog KhuranaNo ratings yet
- General Chemistry I - Tutorial 1Document5 pagesGeneral Chemistry I - Tutorial 1Khuê Nguyễn ThếNo ratings yet
- Measurement of Crushing Strength of Coal AgglomeraDocument5 pagesMeasurement of Crushing Strength of Coal AgglomeraAnonymous 4PuFzARNo ratings yet
- 9science 9 Force and Laws of MotionDocument27 pages9science 9 Force and Laws of MotionMohammed AadilNo ratings yet
- Sed. 1 Se Perfect Chemistry (Vo.) : CalcuDocument1 pageSed. 1 Se Perfect Chemistry (Vo.) : CalcuAayush ShuklaNo ratings yet
- Chapter 14 - Extraction of MetalsDocument2 pagesChapter 14 - Extraction of MetalsAnosha AminNo ratings yet
- Bai Tap Tieng Anh 7 Bai 10Document10 pagesBai Tap Tieng Anh 7 Bai 10Hươngg NguyễnnNo ratings yet
- Scheme of Work Science Stage 8Document90 pagesScheme of Work Science Stage 8Arjun SrinivasanNo ratings yet
- Glide 6.7. User Manual. Schrödinger PressDocument138 pagesGlide 6.7. User Manual. Schrödinger PressKevin Mego De La CruzNo ratings yet
- Cambridge IGCSE: Combined Science 0653/42Document20 pagesCambridge IGCSE: Combined Science 0653/42septinNo ratings yet
- Chemistry FileDocument9 pagesChemistry Filemrharshityadav01No ratings yet
- Abe 106 - 03Document6 pagesAbe 106 - 03emmanuelNo ratings yet
- Fluids Exp 2Document9 pagesFluids Exp 2Ely ReyesNo ratings yet
- Synthesis and Characterization of Nano Banana Fibre Reinforced Polymer Nano CompositesDocument133 pagesSynthesis and Characterization of Nano Banana Fibre Reinforced Polymer Nano CompositesBoopathi RajaNo ratings yet
- Dielectric Coatings For High Voltage Gas Insulated Switchgear - Dennis Van Der Born PDFDocument306 pagesDielectric Coatings For High Voltage Gas Insulated Switchgear - Dennis Van Der Born PDFAmila FritzgeraldNo ratings yet
- Australia To See Fastest Energy Transition in The World Due To - 2020 - Focus oDocument1 pageAustralia To See Fastest Energy Transition in The World Due To - 2020 - Focus oBagoes IdchaNo ratings yet
- Drinking Water ProcessDocument19 pagesDrinking Water ProcessSafitri EkawatiNo ratings yet
- Classification of Economic: DR S e eDocument2 pagesClassification of Economic: DR S e ekishan kumarNo ratings yet
- USP Method For HPLC: Analysis of MethotrexateDocument2 pagesUSP Method For HPLC: Analysis of MethotrexateIsaac GuerreroNo ratings yet
- 页面提取自-Chemistry for the IB Diploma Coursebook, 2nd EditionDocument1 page页面提取自-Chemistry for the IB Diploma Coursebook, 2nd EditionEshowbooks EbooksNo ratings yet