Professional Documents
Culture Documents
Vsepr Chart
Vsepr Chart
Uploaded by
Success Olamide0 ratings0% found this document useful (0 votes)
6 views1 pageCopyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views1 pageVsepr Chart
Vsepr Chart
Uploaded by
Success OlamideCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
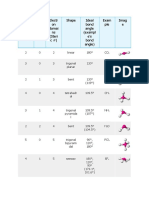
The Complete Table of Hybridization and Geometry
Number of Line, Dash, and Wedge
Electron Hybrid- Electron-Pair Perspective Representation Number of Molecular Bond Polar?
Domains ization Geometry of the Electron-Pair Geometry Lone Pairs Geometry Angles (***)
2 sp Linear 0 Linear 180° No
(two sp hybrid orbitals) A
(2 unhybridized p orbitals are available for π bonding)
3 sp2 Trigonal Planar 0 Trigonal Planar 120° No
(three sp2 hybrid orbitals) A or A 1 Bent < 120° Yes
(1 unhybridized p orbital is available for π bonding)
4 sp3 Tetrahedral 0 Tetrahedral 109.5° No
(four sp3 hybrid orbitals) 1 Trigonal Pyramidal < 109.5° Yes
A
(0 unhybridized p orbitals are leftover; no π bonding) 2 Bent < 109.5° Yes
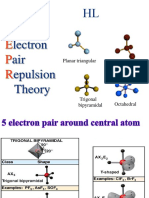
5 sp3d Trigonal Bipyramidal 0 Trigonal Bipyramidal 90° and 120° No
(five sp3d hybrid orbitals) 1 See-Saw < 90° and < 120° Yes
A
(0 unhybridized p orbitals are leftover; no π bonding) 2 T-Shaped < 90° Yes
Note: Contains Axial and Equatorial Positions. 3 Linear 180° No
Lone pairs (if any) go in Equatorial Positions
6 sp3d2 Octahedral 0 Octahedral 90° No
(six sp3d2 hybrid orbitals)
A or A 1 Square Pyramidal < 90° Yes
(0 unhybridized p orbitals are leftover; no π bonding) 2 Square Planar 90° No
Number of Electron Domains (or "Number of electron pairs") = (Number of Other Atoms Something is Bonded To) + (Number of Lone Pairs)
Hybrid orbitals are used to form σ bonds and to hold lone pairs of electrons.
Unhybridized p orbitals are used to form π bonds.
***Note: "Polar?" refers to a molecule in which all terminal atoms are the same. In general, if terminal atoms are different, the molecule will be polar.
You might also like
- Personal Statement For USTCDocument2 pagesPersonal Statement For USTCSabir Ali50% (2)
- 5cis 35 P 41,667.00Document15 pages5cis 35 P 41,667.00jenixson tamondongNo ratings yet
- HW 9 SolutionDocument5 pagesHW 9 SolutionJuan DavidNo ratings yet
- Bondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eDocument3 pagesBondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eaadhyaNo ratings yet
- Vsepr-HlDocument25 pagesVsepr-HlRyan BoukaaNo ratings yet
- Molecular GeometryDocument1 pageMolecular GeometryIsraClarkeNo ratings yet
- Chemical Bonding Day 06Document19 pagesChemical Bonding Day 06S MishraNo ratings yet
- STM 001 Reviewer 1Document10 pagesSTM 001 Reviewer 1Armhay Loraine DuavezNo ratings yet
- 4BCh07 (Basic Properties of Circles 2)Document25 pages4BCh07 (Basic Properties of Circles 2)api-3826695100% (1)
- OriginalDocument28 pagesOriginalTushar BadheNo ratings yet
- Trigonometry ReviewDocument13 pagesTrigonometry ReviewAntonio DionisioNo ratings yet
- 16 JulyDocument19 pages16 JulySubhash YadavNo ratings yet
- Molecular ShapeDocument1 pageMolecular ShapeNUR DEENA KHALID KM-PensyarahNo ratings yet
- Worktable For Shape and PolarityDocument2 pagesWorktable For Shape and PolarityDestinee LegendsNo ratings yet
- Molecular Orbitals PDFDocument6 pagesMolecular Orbitals PDFGeraldNo ratings yet
- Vsepr Table PDFDocument1 pageVsepr Table PDFlucasNo ratings yet
- sin (θ) = a ÷ c cos (θ) = b ÷ c tan (θ) = a ÷ b θ: c po en us e)Document1 pagesin (θ) = a ÷ c cos (θ) = b ÷ c tan (θ) = a ÷ b θ: c po en us e)NikuRikuNo ratings yet
- PreCal 2nd Periodical ReviewerDocument9 pagesPreCal 2nd Periodical ReviewerCastillo Kiefer AnthonyNo ratings yet
- VSEPR TableDocument1 pageVSEPR TableAudrey HizonNo ratings yet
- UNIT 3 Trigonometry (Angles)Document5 pagesUNIT 3 Trigonometry (Angles)Billy Jasper DomingoNo ratings yet
- Edmet f5Document170 pagesEdmet f5adamNo ratings yet
- h=rcos θ ∞: Plane GeometryDocument7 pagesh=rcos θ ∞: Plane GeometryPhreetzi ÜnseenNo ratings yet
- Fundamental Ideas and Measuring AnglesDocument8 pagesFundamental Ideas and Measuring AnglesDebjani MondalNo ratings yet
- Unit Circle in Depth HandoutDocument6 pagesUnit Circle in Depth HandoutFayzaNo ratings yet
- 08 HybridizationPolarity PDFDocument22 pages08 HybridizationPolarity PDFROSEMARIE ONGNo ratings yet
- Geometry of Molecules ChartDocument6 pagesGeometry of Molecules ChartShamsiNo ratings yet
- Azimuths CoordinatesDocument13 pagesAzimuths CoordinatesTamer AbuaitaNo ratings yet
- Understanding Quadrilaterals: PolygonsDocument12 pagesUnderstanding Quadrilaterals: PolygonsBharatNo ratings yet
- Chapter 10 TrigonometryDocument35 pagesChapter 10 TrigonometryNay ChiNo ratings yet
- Notes 5Document10 pagesNotes 5kv8nrv5kdzNo ratings yet
- SadsddsadwaddwdaDocument23 pagesSadsddsadwaddwdaredlegend606No ratings yet
- Chapter 2Document2 pagesChapter 2Jenefer GwmpesawNo ratings yet
- Properties of 2D Shapes - AnswersDocument1 pageProperties of 2D Shapes - Answerscloud scapeNo ratings yet
- General Chemistry 1 Qt. 2 Week 4Document12 pagesGeneral Chemistry 1 Qt. 2 Week 4Nina Reca OmisolNo ratings yet
- CE G2 Module No. 4 Plane and Solid GeometryDocument2 pagesCE G2 Module No. 4 Plane and Solid GeometryDaniela CaguioaNo ratings yet
- Add Maths F5-CHP 1Document13 pagesAdd Maths F5-CHP 1ISMADI SURINNo ratings yet
- Chapter4trigonometry 28versipelajar 29Document18 pagesChapter4trigonometry 28versipelajar 29wan hazirahNo ratings yet
- Form 5 Add MathsDocument57 pagesForm 5 Add MathslimchiayinNo ratings yet
- 5 PDFDocument56 pages5 PDFdNo ratings yet
- 6.1 - Radian Measure and Arc Length Math 30-1Document14 pages6.1 - Radian Measure and Arc Length Math 30-1Math 30-1 EDGE Study Guide Workbook - by RTD LearningNo ratings yet
- SL3.4 The Circle and Radian MeasureDocument16 pagesSL3.4 The Circle and Radian Measurehannah1027kangNo ratings yet
- 8-1 Measurement of Arcs (Presentation)Document13 pages8-1 Measurement of Arcs (Presentation)Sandra Miller100% (1)
- Success Mathematics SPM Free ChapterDocument22 pagesSuccess Mathematics SPM Free ChapterDaryl Lls100% (1)
- Lewis StructureDocument1 pageLewis Structureits aryamNo ratings yet
- 01 Unit Circle and Circular FunctionsDocument21 pages01 Unit Circle and Circular FunctionsJoseph Christopher ButaslacNo ratings yet
- Radians Area of Sector and Arcs Area of Triangle Sine and Cosine RulesDocument24 pagesRadians Area of Sector and Arcs Area of Triangle Sine and Cosine RulesHubert SzeNo ratings yet
- AppendicesDocument9 pagesAppendicesخالدمحمدNo ratings yet
- Electron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles ExampleDocument2 pagesElectron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles ExampleRichamille Ann RicaforteNo ratings yet
- Chap 5 Control System I - Web 2Document16 pagesChap 5 Control System I - Web 2吳晨瑋No ratings yet
- LAS Physical-Science Week2Document11 pagesLAS Physical-Science Week2Shekaina Faith Cuizon LozadaNo ratings yet
- Molecular ModelDocument1 pageMolecular ModelPedro SuyuNo ratings yet
- TrigonometryDocument18 pagesTrigonometryCaitlyn GonzalesNo ratings yet
- Congruency: Mathematics Grade 9Document12 pagesCongruency: Mathematics Grade 9012 Ni Putu Devi AgustinaNo ratings yet
- Reduction To Functions of Positive Acute AnglesDocument8 pagesReduction To Functions of Positive Acute Anglesx seyiNo ratings yet
- Importent FormulasDocument158 pagesImportent FormulasmeseretNo ratings yet
- 12 Trigonometry Basic CalculusDocument18 pages12 Trigonometry Basic Calculusaechel writesNo ratings yet
- .Trashed 1667147460 Circle Theorems Poster 2Document1 page.Trashed 1667147460 Circle Theorems Poster 2Marinela StefanNo ratings yet
- PRECAL Final Module 1 4Document41 pagesPRECAL Final Module 1 4Glen MillarNo ratings yet
- It Should Be Less Than R /3 or 10mDocument5 pagesIt Should Be Less Than R /3 or 10mMawan BentzNo ratings yet
- 1. Angle Measure (Degrees and Radians) : Ab=Oa =Ob α= AB rDocument6 pages1. Angle Measure (Degrees and Radians) : Ab=Oa =Ob α= AB rdahlai dahlia oktaviani gintingNo ratings yet
- Lecture 1.3.1 Angles & RadianDocument62 pagesLecture 1.3.1 Angles & RadianMohd Haffiszul Bin Mohd SaidNo ratings yet
- Day 2Document4 pagesDay 2Tarun KumarNo ratings yet
- Upsc Contact NoDocument2 pagesUpsc Contact NoVishalPandeyNo ratings yet
- Formula Booklet CHE572 Sept2019Document21 pagesFormula Booklet CHE572 Sept2019Mohd Yashfi YunusNo ratings yet
- NR-310804 - Mass Transfer Operations - IDocument8 pagesNR-310804 - Mass Transfer Operations - ISrinivasa Rao G100% (1)
- Duct Design STDocument7 pagesDuct Design STmiladparsmanNo ratings yet
- R7222301 Mass Transfer & SeparationDocument2 pagesR7222301 Mass Transfer & SeparationsivabharathamurthyNo ratings yet
- 02 Analysis of Faults Notes PDFDocument38 pages02 Analysis of Faults Notes PDFJi Chou ChenNo ratings yet
- Quantum Jumping - Shift Your Reality in Big, Positive WaysDocument12 pagesQuantum Jumping - Shift Your Reality in Big, Positive WaysSatish50% (2)
- Coplanar Force ResultantsDocument18 pagesCoplanar Force ResultantsTayabaNo ratings yet
- Force and Motion - 1Document31 pagesForce and Motion - 1yasvialan ariantaNo ratings yet
- Bio201 CM05Document14 pagesBio201 CM05Georges MaaloufNo ratings yet
- Motionless Electromagnetic GeneratorDocument12 pagesMotionless Electromagnetic GeneratorRajesh Chary100% (2)
- Common Engineering Design Conversion FactorsDocument7 pagesCommon Engineering Design Conversion Factorsiran1362No ratings yet
- Journals-5Document106 pagesJournals-5Rajenthar MechNo ratings yet
- Friction Factor For Turbulent Pipe FlowDocument16 pagesFriction Factor For Turbulent Pipe Flowyu won hoNo ratings yet
- P750JDocument1 pageP750JTan Chen TatNo ratings yet
- Capacitors 2: Answer: ExplanationDocument12 pagesCapacitors 2: Answer: ExplanationVia Marie MesaNo ratings yet
- Lec 1 - SuperelevationDocument24 pagesLec 1 - SuperelevationMohamedNo ratings yet
- Design: Ductile of SteelDocument14 pagesDesign: Ductile of SteelVineeth PvNo ratings yet
- HydrostaticStabilityNutshell 220Document16 pagesHydrostaticStabilityNutshell 220mehdimanNo ratings yet
- NOCDocument11 pagesNOCVedam Shanmukha HrushikeshNo ratings yet
- Density LabDocument6 pagesDensity LabfadyaNo ratings yet
- 2009 IB Physics - SL Fields MSDocument7 pages2009 IB Physics - SL Fields MSGajendraNo ratings yet
- Chapter 8 Two-Dimensional Problem SolutionDocument51 pagesChapter 8 Two-Dimensional Problem Solutiongpskumar22No ratings yet
- PHASM/G 442: 2013: Problem Sheet 2: Please Return To DR Ryan Nichol at The November 14Document2 pagesPHASM/G 442: 2013: Problem Sheet 2: Please Return To DR Ryan Nichol at The November 14Roy VeseyNo ratings yet
- EE110Document2 pagesEE110Varsha VasthaviNo ratings yet
- Lesson 2 Basic Circuit LawsDocument10 pagesLesson 2 Basic Circuit LawsMD HOSSAINNo ratings yet
- ENGG 112 Elements of Engineering II 3 CreditsDocument1 pageENGG 112 Elements of Engineering II 3 CreditsRojan PradhanNo ratings yet