Professional Documents
Culture Documents
Periodic Table
Uploaded by
Alliyah vidanesOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Periodic Table
Uploaded by
Alliyah vidanesCopyright:
Available Formats
CHEMISTRY PRACTICAL NOTES
Test for gases
gas test and test result
ammonia (NH3) turns damp red litmus paper blue
carbon dioxide (CO2) gives white ppt, calcium carbonate, CaCO3 with limewater,
calcium hydroxide, Ca(OH)2
hydrogen (H2) “pops” with a lighted splint
oxygen (O2) relights a glowing splint
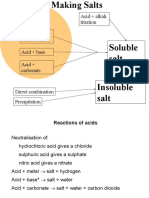
Solubility of salts (with reference to dissolving in water)
Soluble Salt (aq) Insoluble Salts (s)
Can be dissolved in water Cannot be dissolved in water
Carbonates Sodium, potassium, ammonium (SPA) All others
All except
Chlorides Lead(II) chloride, Silver chloride
All except Barium sulfate, Calcium sulfate,
Sulfates Lead(II) sulfate
All Sodium, Potassium, Ammonia salts are soluble (aq).
All Nitrate salts are soluble. (SPAN)
Metal oxides Metal hydroxides
(Note: All metal oxides are insoluble(s). (Note: All metal hydroxides are
insoluble(s) except the following: (SPCA)
Insoluble bases (s) Soluble bases or alkalis (aq)
Magnesium oxide MgO(s) Sodium hydroxide NaOH(aq)
Copper(II) oxide CuO(s) Potassium hydroxide KOH(aq)
Lead(II) oxide PbO(s) Calcium hydroxide Ca(OH)2 (aq)
Ammonium hydroxide NH4OH(aq)
The Mole Salt Preparation
Types of Oxides
You might also like
- Chapter 8: Salts: Flow Chart of Preparation of SaltsDocument7 pagesChapter 8: Salts: Flow Chart of Preparation of SaltsPrincess Ting TingNo ratings yet
- SAlt Preperation - 1Document14 pagesSAlt Preperation - 1youssefelassal2009No ratings yet
- Acids and Bases SummaryDocument2 pagesAcids and Bases SummaryTan Yong KhaiNo ratings yet
- Garam Bab 8Document29 pagesGaram Bab 8ctohNo ratings yet
- Acids, Bases and SaltsDocument4 pagesAcids, Bases and Saltsbubutrain2003No ratings yet
- Chapter 8: SaltsDocument14 pagesChapter 8: SaltsLynn HengNo ratings yet
- Types of OxideDocument1 pageTypes of OxideTamoya ShirleyNo ratings yet
- AcidsDocument3 pagesAcidsPratham GoradiaNo ratings yet
- Solubility RulesDocument1 pageSolubility RulesAdamNo ratings yet
- 3 Experiment ChemistryDocument30 pages3 Experiment ChemistryThangavel SarujanNo ratings yet
- Microsoft Word - Chemsheets GCSE 1191 (Acid, Base or Salt)Document1 pageMicrosoft Word - Chemsheets GCSE 1191 (Acid, Base or Salt)Lanbin CuiNo ratings yet
- Acids RecallDocument4 pagesAcids RecallAssumpta McguckinNo ratings yet
- Acids, Bases, Salts: - Substances That Show Acidic and Basic Properties - Reactions of Acids and Bases - SaltsDocument24 pagesAcids, Bases, Salts: - Substances That Show Acidic and Basic Properties - Reactions of Acids and Bases - SaltsEren MERICNo ratings yet
- Solubility Rules: Strong & in WaterDocument1 pageSolubility Rules: Strong & in WaterChelsea MartinezNo ratings yet
- Salts PreparationDocument7 pagesSalts PreparationCynthia RoneyNo ratings yet
- Notes On SaltsDocument4 pagesNotes On SaltsFelix S100% (1)
- Oxides, Acids, Bases and SaltsDocument8 pagesOxides, Acids, Bases and Salts12&13 SciencesNo ratings yet
- Must Know For Chapter 9 - Salts (And C11 Qualitative Analysis)Document4 pagesMust Know For Chapter 9 - Salts (And C11 Qualitative Analysis)Chaw Wei HengNo ratings yet
- ( (Chapter 8&9 - Acids and Bases, Salts) )Document8 pages( (Chapter 8&9 - Acids and Bases, Salts) )bharadiadishitaNo ratings yet
- Part IV Acids and Alkalis: LQ 01 (Answer) Properties of Acids and AlkalisDocument12 pagesPart IV Acids and Alkalis: LQ 01 (Answer) Properties of Acids and AlkalisCharmine HolmesNo ratings yet
- Booklet On Acid and Base and Redox MSDocument43 pagesBooklet On Acid and Base and Redox MShalahossam8899No ratings yet
- Chapter 8 SALTSDocument75 pagesChapter 8 SALTSSiti Hajar Abd HamidNo ratings yet
- Chemistry F4: Theme 3: Interaction Between Matters Chapter 6: Acid, Base & Salt (6.8 - 6.11)Document29 pagesChemistry F4: Theme 3: Interaction Between Matters Chapter 6: Acid, Base & Salt (6.8 - 6.11)Novah GurulooNo ratings yet
- 3E5NA Sci Chem Qualitative Analysis Notes Student'sDocument19 pages3E5NA Sci Chem Qualitative Analysis Notes Student'sAditi Ravi kaushikNo ratings yet
- Salt 2020 PDFDocument42 pagesSalt 2020 PDFNurulNo ratings yet
- Chapter 8 - Acids, Bases and SaltsDocument16 pagesChapter 8 - Acids, Bases and Saltsjannat amgadNo ratings yet
- Cha 11Document11 pagesCha 11Tun Lin AungNo ratings yet
- 4th Form Qualitative Analysis Sheet Summary SheetDocument2 pages4th Form Qualitative Analysis Sheet Summary SheetFrank MassiahNo ratings yet
- Data Sheet Revision PDFDocument2 pagesData Sheet Revision PDFShifa RizwanNo ratings yet
- BasesDocument45 pagesBasesDinara DzhakishovaNo ratings yet
- Half and Ionic Equations (GCSE)Document31 pagesHalf and Ionic Equations (GCSE)william.ongeri.tutoringNo ratings yet
- Wan Noor Afifah BT Wan YusoffDocument33 pagesWan Noor Afifah BT Wan YusoffThilagavathyNo ratings yet
- Csec Identification of Cations and AnionsDocument6 pagesCsec Identification of Cations and AnionsDarrion BruceNo ratings yet
- Heat Cao S Ho Ca Oh Aq: Hol H G OgDocument12 pagesHeat Cao S Ho Ca Oh Aq: Hol H G OgValyanaNo ratings yet
- Nomenclature: General Chemistry Pro-KnowledgeDocument2 pagesNomenclature: General Chemistry Pro-KnowledgemohammedNo ratings yet
- Solubility Rules Practice WorksheetDocument2 pagesSolubility Rules Practice WorksheetSarah Yetti0% (1)
- Salts FormationDocument19 pagesSalts FormationUrwa Abdul MannanNo ratings yet
- Half and Ionic Equations (GCSE)Document31 pagesHalf and Ionic Equations (GCSE)william.ongeri.tutoringNo ratings yet
- Ariana's Rules For Solubility of Ionic Compounds in WaterDocument2 pagesAriana's Rules For Solubility of Ionic Compounds in Waterakavi1No ratings yet
- Common Compounds Acids: Chemical Name Chemical Formula Common Name/S CH Cooh CH Cooh H Bo H Co HCL HCN Hno H So 1 Part Hno: 3 Parts HCLDocument3 pagesCommon Compounds Acids: Chemical Name Chemical Formula Common Name/S CH Cooh CH Cooh H Bo H Co HCL HCN Hno H So 1 Part Hno: 3 Parts HCLFrederick FranciscoNo ratings yet
- Chemistry: Form 4-Chapter 8Document7 pagesChemistry: Form 4-Chapter 8Muhamad AazrilNo ratings yet
- C2710 Qualitative Analysis of Group 2 Metal IonsDocument4 pagesC2710 Qualitative Analysis of Group 2 Metal IonssispulieNo ratings yet
- Chemistry Chapter 1.exercise 1ADocument28 pagesChemistry Chapter 1.exercise 1AAsifNo ratings yet
- Valency SheetDocument3 pagesValency SheetBex JacobsNo ratings yet
- Notes Updates SaltsDocument32 pagesNotes Updates SaltsLim Jing YeeNo ratings yet
- Grade 10 Chemistry Week 12 Lesson 1Document4 pagesGrade 10 Chemistry Week 12 Lesson 1nesiaroberts903No ratings yet
- Che - 91911 Identification of Al3+Document1 pageChe - 91911 Identification of Al3+Jo StandleyNo ratings yet
- Solubility TableDocument1 pageSolubility TableNontando MendieNo ratings yet
- 10 Nature of Oxides2Document28 pages10 Nature of Oxides2James WongNo ratings yet
- Form 2 Introduction To SaltsDocument11 pagesForm 2 Introduction To Saltsemilykwamboka500No ratings yet
- Naming ReviewDocument1 pageNaming Reviewlee_magsinoNo ratings yet
- Symbols and Names For Common Polyatomic IonsDocument1 pageSymbols and Names For Common Polyatomic IonsElixirNo ratings yet
- Chapter 6 Acids, Bases and SaltsDocument32 pagesChapter 6 Acids, Bases and SaltsAnne Marie Ya Jie GOHNo ratings yet
- Form Acid When Dissolved in WaterDocument2 pagesForm Acid When Dissolved in WaterErshad HussainNo ratings yet
- Acids, Bases and SaltsDocument9 pagesAcids, Bases and SaltsShalom LogosNo ratings yet
- OXIDES (Metals & Non-Metals)Document4 pagesOXIDES (Metals & Non-Metals)gauri guptaNo ratings yet
- 50 Ways To Name Your Compound: Assignment: Write The Corresponding Name or Formula For Each of The FollowingDocument1 page50 Ways To Name Your Compound: Assignment: Write The Corresponding Name or Formula For Each of The FollowingRavenia Ghani PutriNo ratings yet
- Chemistry Chemical NamesDocument3 pagesChemistry Chemical NamesSaurabh MehtaNo ratings yet
- List of Copper Salts - WikipediaDocument6 pagesList of Copper Salts - WikipediababuNo ratings yet
- SS Sotong Guide Issue 3 - N Level SyllabusDocument22 pagesSS Sotong Guide Issue 3 - N Level SyllabusAlliyah vidanesNo ratings yet
- How To Handle SBQ - AESDocument4 pagesHow To Handle SBQ - AESAlliyah vidanesNo ratings yet
- Open SS Cheat Sheets For SRQ (B) - CompiledDocument23 pagesOpen SS Cheat Sheets For SRQ (B) - CompiledAlliyah vidanesNo ratings yet
- Open SS Cheat Sheets For SRQ (B) - CompiledDocument23 pagesOpen SS Cheat Sheets For SRQ (B) - CompiledAlliyah vidanesNo ratings yet
- Tourism KQ2 NotesDocument8 pagesTourism KQ2 NotesAlliyah vidanesNo ratings yet
- Tectonic Notes (Compiled Notes) 2019Document29 pagesTectonic Notes (Compiled Notes) 2019Alliyah vidanesNo ratings yet
- Tourism KQ1 How Does The Nature of Tourism Vary From Place To Place?Document7 pagesTourism KQ1 How Does The Nature of Tourism Vary From Place To Place?Alliyah vidanesNo ratings yet
- K-Drama RatesDocument2 pagesK-Drama RatesAlliyah vidanesNo ratings yet
- CoursesDocument1 pageCoursesAlliyah vidanesNo ratings yet
- Bundled NotesDocument189 pagesBundled NotesAlliyah vidanesNo ratings yet
- Tanglin Secondary School Preliminary Examination 2020: Secondary 4 Express & 5 Normal (Academic)Document7 pagesTanglin Secondary School Preliminary Examination 2020: Secondary 4 Express & 5 Normal (Academic)Alliyah vidanesNo ratings yet