Professional Documents
Culture Documents
Acids, Bases and Salts
Uploaded by
bubutrain2003Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Acids, Bases and Salts
Uploaded by
bubutrain2003Copyright:
Available Formats
Acids, bases & salts 1
ACIDS, BASES & SALTS
ACIDS Release H+ ions (protons) in aqueous solution
Hydrochloric HCl —> H+(aq) + Cl¯(aq) MONOPROTIC 1 replaceable H
Nitric HNO3 —> H+(aq) + NO3¯(aq) MONOPROTIC 1 replaceable H
Sulphuric H2SO4 —> 2H+(aq) + SO42-(aq) DIPROTIC 2 replaceable H’s
Ethanoic CH3COOH(aq) CH3COO¯(aq) + H+(aq) A WEAK ACID
BASES React with acids by accepting H+ ions (protons) to form salts
carbonates K2CO3 MgCO3 CuCO3
hydrogencarbonates NaHCO3
metal oxides MgO ZnO CuO
metal hydroxides NaOH KOH Ca(OH)2
ammonia NH3
Knockhardy Publishing
ALKALIS SOLUBLE BASES which release OH¯ (hydroxide ions) in aqueous solution
Soluble metal oxides Na2O + H2O(l) —> 2Na+(aq) + 2OH¯(aq)
Soluble metal hydroxides NaOH —> Na+(aq) + OH¯(aq)
sodium hydroxide
KOH —> K+(aq) + OH¯(aq)
potassium hydroxide
Aqueous ammonia NH3 (aq) + H2O(l) NH4+(aq) + OH¯(aq)
SALTS Formed from the reaction between acids and bases
hydrochloric acid makes CHLORIDES
nitric acid makes NITRATES
sulphuric acid makes SULPHATES / HYDROGENSULPHATES
© KNOCKHARDY PUBLISHING 2015
2 Acids, bases & salts
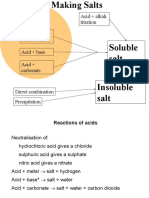
SALT FORMATION
Formation A salt is produced when the H+ ion of an acid is replaced by...
a metal ion or the ammonium ion NH4+
SUMMARY
Acids react with...
metals to give a salt + hydrogen
oxides of metals a salt + water
hydroxides of metals a salt + water
carbonates a salt + water + carbon dioxide
hydrogencarbonates a salt + water + carbon dioxide
ammonia an ammonium salt
Water of
crystallisation • loosely bonded water molecules attached to salts
Knockhardy Publishing
CuSO4.5H2O FeSO4.7H2O Na2CO3.10H2O
• the water can be driven off by heating
e.g. CuSO4.5H2O(s) ———> CuSO4(s) + 5H2O(l)
HYDRATED copper(II) sulphate ANHYDROUS copper(II) sulphate
BLUE CRYSTALS WHITE POWDER
PROPERTIES AND REACTIONS OF HYDROCHLORIC ACID
Hydrochloric acid is a typical acid; in dilute aqueous solution HCl —> H+(aq) + Cl¯(aq)
Hydrogen chloride is a colourless gas; it is a poor conductor of
electricity because there are no free electrons or ions present. It
has no action on dry litmus paper because there are no
aqueous hydrogen ions present.
In water, the covalent hydrogen chloride molecules dissociate
into ions. The solution now conducts electricity showing ions are
present. For each hydrogen chloride molecule that dissociates
one hydrogen ion and one chloride ion are produced. The The dissociation of hydrogen chloride
solution turns litmus paper red because of the into ions when put in water
presence of the H+(aq) ion.
Appearance Bonding and formula Conductivity Dry litmus
hydrogen chloride colourless gas covalent molecule HCl(g) poor no reaction
hydrochloric acid colourless soln. aqueous ions HCl(aq) good goes red
© KNOCKHARDY PUBLISHING 2015
Acids, bases & salts 3
THE REACTIONS OF ACIDS
Metals magnesium + hydrochloric acid ——> magnesium chloride + hydrogen
Mg(s) + 2HCl(aq) ——> MgCl2(aq) + H2(g)
Mg(s) + 2H+(aq) + 2Cl¯(aq) ——> Mg2+(aq) + 2Cl¯(aq) + H2(g)
cancel ions Mg(s) + 2H+(aq) ——> Mg2+(aq) + H2(g)
Basic
Oxides copper(II) oxide + hydrochloric acid ——> copper(II) chloride + water
CuO(s) + 2HCl(aq) ——> CuCl2(aq) + H2O(l)
Cu2+O2-(s) + 2H+(aq) + 2Cl¯(aq) ——> Cu2+(aq) + 2Cl¯(aq) + H2O(l)
cancel ions O2- + 2H+(aq) ——> H2O(l)
Knockhardy Publishing
Alkalis sodium hydroxide + hydrochloric acid ——> sodium chloride + water
NaOH(aq) + HCl(aq) ——> NaCl(aq) + H2O(l)
Na+(aq) + OH¯(aq) + H+(aq) + Cl¯(aq) ——> Na+(aq) + Cl¯(aq) + H2O(l)
cancel ions H+(aq) + OH¯(aq) ——> H2O(l)
Carbonates calcium + hydrochloric ——> calcium + carbon + water
carbonate acid chloride dioxide
CaCO3(s) + 2HCl(aq) ——> CaCl2(aq) + CO2(g) + H2O(l)
Ca2+CO32-(s) + 2H+(aq) + 2Cl¯(aq) ——> Ca2+(aq) + 2Cl¯(aq) + CO2(g) + H2O(l)
cancel ions CO32- + 2H+(aq) ——> CO2(g) + H2O(l)
Hydrogencarbonates H+(aq) + HCO3¯ ——> CO2(g) + H2O(l)
© KNOCKHARDY PUBLISHING 2015
4 Acids, bases & salts
Q.1 Write the formulae for...
a) zinc chloride b) zinc sulphate
c) magnesium sulphate d) magnesium nitrate
e) aluminium sulphate f) potassium carbonate
g) ammonium chloride h) ammonium sulphate
Q.2 Write balanced equations for the reactions between...
a) zinc and dilute hydrochloric acid
b) zinc and dilute sulphuric acid
c) magnesium oxide and dilute sulphuric acid
Knockhardy Publishing
d) zinc oxide and dilute nitric acid
e) potassium hydroxide and dilute hydrochloric acid
f) potassium hydroxide and dilute sulphuric acid
g) magnesium carbonate and dilute sulphuric acid
h) ammonia solution and dilute hydrochloric acid
i) ammonia solution and dilute sulphuric acid
Q.3 Calculate the percentage of water (by mass) in the following hydrated salts;
a) CuSO4.5H2O
b) Na2CO3.10H2O
© KNOCKHARDY PUBLISHING 2015
You might also like
- Part IV Acids and Bases NotesDocument45 pagesPart IV Acids and Bases NotesHon KwanNo ratings yet
- Acids, Bases and SaltsDocument9 pagesAcids, Bases and SaltsShalom LogosNo ratings yet
- 8.2 Formulae Equations and Amount Edexcel 15 17Document3 pages8.2 Formulae Equations and Amount Edexcel 15 17Stephan MinhNo ratings yet
- NeutralizationDocument13 pagesNeutralizationbarakahmuluziNo ratings yet
- Chapter 6 Acids, Bases and SaltsDocument32 pagesChapter 6 Acids, Bases and SaltsAnne Marie Ya Jie GOHNo ratings yet
- Acid BaseDocument24 pagesAcid BaseyusmahanimNo ratings yet
- Topic 7Document16 pagesTopic 7nighat12No ratings yet
- Oxides, Acids, Bases and SaltsDocument8 pagesOxides, Acids, Bases and Salts12&13 SciencesNo ratings yet
- Making Salts: Neutralisation ReactionsDocument4 pagesMaking Salts: Neutralisation ReactionsPedro Moreno de SouzaNo ratings yet
- Carbon Carbon Dioxide Carbon Carbon Dioxide: MG MGDocument3 pagesCarbon Carbon Dioxide Carbon Carbon Dioxide: MG MGDSE No WorriesNo ratings yet
- Form 4 Acid, Bases and Salts NotesDocument21 pagesForm 4 Acid, Bases and Salts NotesTamisha JacobsNo ratings yet
- Salts FormationDocument19 pagesSalts FormationUrwa Abdul MannanNo ratings yet
- Acids Bases and SaltsDocument45 pagesAcids Bases and SaltsTejas PagarNo ratings yet
- Zaim NotesDocument5 pagesZaim NotesZafirah SuffianNo ratings yet
- Acids, Bases and Salts Notes: ChemistryDocument20 pagesAcids, Bases and Salts Notes: ChemistryLavanya Priya SathyanNo ratings yet
- ACIDS AND BASES DEFINEDDocument25 pagesACIDS AND BASES DEFINEDyusmahanimNo ratings yet
- Notes Acids and BasesDocument10 pagesNotes Acids and BasesRabia Ashraf - 75828/TCHR/BSSRNo ratings yet
- Acids, Bases and SaltsDocument8 pagesAcids, Bases and Saltsaakashb1918No ratings yet
- [[Chapter 8&9_ Acids and Bases, Salts]]Document8 pages[[Chapter 8&9_ Acids and Bases, Salts]]bharadiadishitaNo ratings yet
- CA DEM Y: Chapter - 2 - NOTESDocument2 pagesCA DEM Y: Chapter - 2 - NOTESvarun puriNo ratings yet
- Chemistry Notes Acids Bases and SaltsDocument7 pagesChemistry Notes Acids Bases and SaltsGouri RajNo ratings yet
- Acids and BasesDocument73 pagesAcids and Basesapi-305909325100% (4)
- 10 TH Acids, Bases, and Salts ChemistryDocument12 pages10 TH Acids, Bases, and Salts ChemistryShabir KhanNo ratings yet
- Acids RecallDocument4 pagesAcids RecallAssumpta McguckinNo ratings yet
- Chem - Acids and Bases and Ionic EquationsDocument23 pagesChem - Acids and Bases and Ionic EquationsYasser AliNo ratings yet
- Final Revision Acids, Bases and Salts (Repaired) PDFDocument13 pagesFinal Revision Acids, Bases and Salts (Repaired) PDFRawan Abd ElaatyNo ratings yet
- Chemistry F4 SaltsDocument13 pagesChemistry F4 Saltscivichitam18No ratings yet
- Acids and Bases SummaryDocument2 pagesAcids and Bases SummaryTan Yong KhaiNo ratings yet
- Acids, Bases, Salts: - Substances That Show Acidic and Basic Properties - Reactions of Acids and Bases - SaltsDocument24 pagesAcids, Bases, Salts: - Substances That Show Acidic and Basic Properties - Reactions of Acids and Bases - SaltsEren MERICNo ratings yet
- Acids, Bases & Salts: IndicatorsDocument7 pagesAcids, Bases & Salts: IndicatorsView TubeNo ratings yet
- Acids, Bases and Salts EssentialsDocument4 pagesAcids, Bases and Salts EssentialsAarush GuptaNo ratings yet
- Acids, Bases & Salts: Properties and ClassificationDocument4 pagesAcids, Bases & Salts: Properties and ClassificationView TubeNo ratings yet
- Acids, Bases and Salts: Key ConceptsDocument19 pagesAcids, Bases and Salts: Key ConceptsRoopika Chaudhary CherukuriNo ratings yet
- Acids - 2Document2 pagesAcids - 2Anushka ShendageNo ratings yet
- Solution 805196Document4 pagesSolution 805196scNo ratings yet
- Notes On SaltsDocument4 pagesNotes On SaltsFelix S100% (1)
- REACTION OF METALS WITH ACIDS AND SALTSDocument3 pagesREACTION OF METALS WITH ACIDS AND SALTSDarshanaK 728714No ratings yet
- Acids Bases and Salts 100l 1Document5 pagesAcids Bases and Salts 100l 1Michael EhondorNo ratings yet
- 3E5NA Sci Chem Qualitative Analysis Notes Student'sDocument19 pages3E5NA Sci Chem Qualitative Analysis Notes Student'sAditi Ravi kaushikNo ratings yet
- Acids: Common Acids in Daily LifeDocument47 pagesAcids: Common Acids in Daily LifeElanIe InspiritNo ratings yet
- Reactions in Aqueous SolutionDocument48 pagesReactions in Aqueous SolutionDavid MaranzhyanNo ratings yet
- Part IV Acids and Alkalis: LQ 01 (Answer) Properties of Acids and AlkalisDocument12 pagesPart IV Acids and Alkalis: LQ 01 (Answer) Properties of Acids and AlkalisCharmine HolmesNo ratings yet
- Acid Bases and Salts Igcse Chemistry 0620Document15 pagesAcid Bases and Salts Igcse Chemistry 0620Aminah ShahzadNo ratings yet
- A Closer Look at Acids: Chapter - 9.2Document13 pagesA Closer Look at Acids: Chapter - 9.2goodboyhokyaNo ratings yet
- Acids: Understand The Chemistry and Remember The EquationsDocument1 pageAcids: Understand The Chemistry and Remember The EquationsAnurag AwasthiNo ratings yet
- 10th SCIENCE (English medium) must do (4) (4)Document63 pages10th SCIENCE (English medium) must do (4) (4)anshu26stNo ratings yet
- ACIDS and BASES Notes & WorksheetDocument9 pagesACIDS and BASES Notes & WorksheetAdeenaNo ratings yet
- Chemistry Acid BasesDocument19 pagesChemistry Acid BasesYusra RasoolNo ratings yet
- Acid & BasesDocument4 pagesAcid & BasesWaaz AmjadNo ratings yet
- CLASS X CHEMISTRY Solution-989564Document6 pagesCLASS X CHEMISTRY Solution-989564abiniveshofficial4708No ratings yet
- Chemical ReactionsDocument2 pagesChemical ReactionsKrydztom UyNo ratings yet
- Evolution of Gas:: Other Endothermic ReactionsDocument7 pagesEvolution of Gas:: Other Endothermic Reactionssara parkNo ratings yet
- Acids and BasesDocument98 pagesAcids and BasesLaziNo ratings yet
- S4 Chemistry Summary NotesDocument21 pagesS4 Chemistry Summary NotesbonnealbineNo ratings yet
- CH 15 Acids Bases and SaltsDocument104 pagesCH 15 Acids Bases and SaltsDaevy Toribio AmdosNo ratings yet
- Acid Bases & SaltsDocument7 pagesAcid Bases & Saltssհízα ƒαlαҡNo ratings yet
- Copper Cycle Report SP16Document12 pagesCopper Cycle Report SP16BirobaNo ratings yet
- Acids, Bases and SaltsDocument15 pagesAcids, Bases and SaltsSarah MariaNo ratings yet
- Acids Bases & SaltsDocument19 pagesAcids Bases & SaltsSillolwazi BroughNo ratings yet
- CIE Chemistry A-Level: Practicals For Papers 3 and 5Document7 pagesCIE Chemistry A-Level: Practicals For Papers 3 and 5bubutrain2003No ratings yet
- Haber process conditionsDocument1 pageHaber process conditionsbubutrain2003No ratings yet
- Paper 5 - Analysis Conclusions and Evaluation PDFDocument6 pagesPaper 5 - Analysis Conclusions and Evaluation PDFTan Yan YingNo ratings yet
- Percentage ErrorDocument6 pagesPercentage ErrormalakmounirNo ratings yet
- CIE Chemistry A-Level Practicals for Papers 3 and 5 Gases EvolvedDocument3 pagesCIE Chemistry A-Level Practicals for Papers 3 and 5 Gases EvolvedHuzefa shaikhNo ratings yet
- 08entro PDFDocument0 pages08entro PDFNorbertus Krisnu PrabowoNo ratings yet
- 1 PDFDocument5 pages1 PDFHoward YuNo ratings yet
- Element Atom Molecule: Useful DefinitionsDocument7 pagesElement Atom Molecule: Useful DefinitionsSachitra WijethungaNo ratings yet
- CIE Chemistry A Level: 13: Nitrogen and SulfurDocument4 pagesCIE Chemistry A Level: 13: Nitrogen and Sulfurbubutrain2003No ratings yet
- An Introduction To The Chemistry of Transition ElementsDocument13 pagesAn Introduction To The Chemistry of Transition Elementsbubutrain2003No ratings yet
- 15 Tmet 2Document8 pages15 Tmet 2Ibrahim SalimNo ratings yet
- Periodicity: Periodic TableDocument5 pagesPeriodicity: Periodic TableYamac SamaniNo ratings yet
- Shapes of Molecules and Ions PDFDocument2 pagesShapes of Molecules and Ions PDFYamac SamaniNo ratings yet
- Group 7 - Halogens, Halide Ions and UsesDocument5 pagesGroup 7 - Halogens, Halide Ions and Usesbubutrain2003No ratings yet
- 5 Aldehydes and Ketones-Structure and PreparationDocument41 pages5 Aldehydes and Ketones-Structure and PreparationKeshav JoshiNo ratings yet
- Chemical List 2018Document6 pagesChemical List 2018Jowie Lica CabaccanNo ratings yet
- Japanese Excluded List of PigmentsDocument31 pagesJapanese Excluded List of PigmentsEllen De Oliveira DeNo ratings yet
- TRẮC NGHIỆM HÓA HỮU CƠDocument5 pagesTRẮC NGHIỆM HÓA HỮU CƠLêPhạmTrungNhơnNo ratings yet
- Lap ResepDocument198 pagesLap ResepStien DwinyNo ratings yet
- PriceList IMD Update Okt'22Document2 pagesPriceList IMD Update Okt'22MDafaANo ratings yet
- Vermont Pub & Brewery's Water Witch Water Treatment GuideDocument3 pagesVermont Pub & Brewery's Water Witch Water Treatment GuideRiyanNo ratings yet
- Conclusion and Recommendations3Document2 pagesConclusion and Recommendations3Lara Melissa Orense100% (7)
- JT BAKER CatalogDocument73 pagesJT BAKER CatalogwardoyogussanNo ratings yet
- Química Orgánica: Docente: Alba ChaparroDocument2 pagesQuímica Orgánica: Docente: Alba ChaparroCamila MendezNo ratings yet
- Invernol 2021Document18 pagesInvernol 2021Victor JustinianoNo ratings yet
- Word Equation PracticeDocument20 pagesWord Equation PracticePrimoNo ratings yet
- Simple List AlphaDocument42 pagesSimple List AlphaRonald Ivan WijayaNo ratings yet
- 2016 AfterSun-Cushion FoundationDocument2 pages2016 AfterSun-Cushion FoundationIra Murti SariNo ratings yet
- Chemistry Assessment 1Document12 pagesChemistry Assessment 1Nayyir Mumtasir Rahman 2323059047No ratings yet
- Acids and Bases: Test Yourself 11.1 (Page 189)Document3 pagesAcids and Bases: Test Yourself 11.1 (Page 189)何小霞No ratings yet
- Daftar Obat FahrenheitDocument15 pagesDaftar Obat FahrenheitRahmah Gadis Wartania Putri50% (2)
- Jenis Fatty Alcohol (Stearyl Alcohol)Document26 pagesJenis Fatty Alcohol (Stearyl Alcohol)Rika Dian FitrianaNo ratings yet
- Activity Sheets On Chemical NomenclatureDocument3 pagesActivity Sheets On Chemical NomenclatureSam Ashley Dela CruzNo ratings yet
- Basic Chemical Reaction WorksheetDocument12 pagesBasic Chemical Reaction Worksheettranquil_452889939No ratings yet
- Soda Escamas - Modificado VeterquimicaDocument1 pageSoda Escamas - Modificado Veterquimicajuan aguileraNo ratings yet
- 2 ADocument45 pages2 AShyam SundarNo ratings yet
- CHEM 221 Exercise Book Unit II-1Document5 pagesCHEM 221 Exercise Book Unit II-1bacha01No ratings yet
- Chapter Fourteen Topic Nomenclature of E PDFDocument44 pagesChapter Fourteen Topic Nomenclature of E PDFy100% (2)
- WTP chemical inventory list with stock levelsDocument1 pageWTP chemical inventory list with stock levelsReynaldi Be TambunanNo ratings yet
- Tablas de Nomenclatura Con S N PDocument13 pagesTablas de Nomenclatura Con S N PLucero RamirezNo ratings yet
- Chemical Resistance Guide: Chemicals ChemicalsDocument13 pagesChemical Resistance Guide: Chemicals ChemicalsendyNo ratings yet
- Vademecum Valesalud 201401Document42 pagesVademecum Valesalud 201401petacosNo ratings yet
- ReaktorDocument10 pagesReaktoralmiraNo ratings yet
- OzonolysisDocument1 pageOzonolysisThu NguyenNo ratings yet

















![[[Chapter 8&9_ Acids and Bases, Salts]]](https://imgv2-1-f.scribdassets.com/img/document/726769244/149x198/8df8f4f441/1714097780?v=1)