Professional Documents
Culture Documents
Gr11 Rev Ch05 02 QnA
Uploaded by
Aidan0 ratings0% found this document useful (0 votes)
1 views2 pagesOriginal Title
Gr11_Rev_Ch05_02_QnA
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
1 views2 pagesGr11 Rev Ch05 02 QnA
Uploaded by
AidanCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
Dr Vince Grade 11 Physics Detailed Revisions [Ch5-02] 1
G11-C05-Q02-A: Fill in the blanks.
1. The thermal capacity per unit mass of a substance is the ____________ of the substance.
2. The SI unit of the specific heat capacity is ___________.
3. The units of the specific heat capacity other than the SI unit are __________
4. The specific heat capacity of 10 kg metal X is 50x J kg-1 K-1. The specific heat capacity of 20 kg metal
X is ________.
5. The thermal capacity of 10 kg metal X is 50x J K-1. The thermal capacity of 20 kg metal X is _______.
G11-C05-Q02-B: Say True (or) False.
1. The thermal capacity depends on the mass of the material.
2. The thermal capacity depends on the type of the material.
3. The quantity of heat required to increase the temperature of a mass m of a certain material is
proportional to the temperature change.
4. The quantity of heat required to increase the temperature of a mass m of a certain material is
proportional to the mass of the material.
5. The quantity of heat required to increase the temperature of a mass m of a certain material is
proportional to the type of the material.
6. The specific heat has different values for different materials.
7. When Q and T are positive, heat enters the object and its temperature increases.
8. When Q and T are negative, heat leaves the object and its temperature decreases.
9. Q does not represent a change in the amount of heat contained in an object.
10. Heat is always energy in transit as a result of a temperature difference.
11. There is no such thing as “the amount of heat in an object.”
12. The specific heat of a material always depends somewhat on the initial temperature and the
temperature interval.
13. The molar mass of any substance, denoted by M, is the mass per mole.
14. 1 mole of water has a mass of 18.0 g.
15. Measurements of specific heats capacities for solid materials are usually made at constant atmospheric
pressure.
16. The molar mass of any substance is the mass per mole.
17. Heat is energy in transit to or from an object, not the energy residing in the object.
18. Measurements of specific heat capacities for solid materials are usually made at constant atmospheric
pressure; the specific heat capacity at constant pressure is denoted by cp.
19. For a gas it is usually easier to keep the substance in a container with constant volume; the specific
heat capacity at constant volume is denoted by cV.
20. For a given substance, cV and cp are different.
2 Grade 11 Physics Detailed Revisions [Ch5-02] Dr Vince
21. If the system can expand while heat is added, there is additional energy exchange through the
performance of work by the system on its surroundings.
22. If the volume is constant, the system does no work.
23. The molar heat capacities for most elemental solids are about the same: about 25 J mol-1 K-1.
24. The number of atoms in 1 mole is the same for all elemental substances.
25. On a per atom basis, about the same amount of heat is required to raise the temperature of each of
these elements by a given amount, even though the masses of the atoms are very different.
26. The heat required for a given temperature increase depends only on how many atoms the sample
contains, not on the mass of an individual atom.
27. When a gas expands, it pushes outward on its boundary surfaces as they move outward.
28. An expanding gas always does positive work.
29. When the force has a component in the same direction as the displacement, the work is positive
30. When the force has a component opposite to the displacement, the work is negative.
31. When the force is perpendicular to the displacement, the work done by the force is zero.
32. Work is positive when a system expands.
33. When a system is compressed, its volume decreases and it does negative work on its surroundings.
34. The heat gained or lost by an object as its temperature changes depends on the mass, the change in
temperature, and the specific heat of the substance.
G11-C05-Q02-C: Short Questions.
1. Define specific heat capacity. Does it depend on mass of the substance? Does it depend on temperature of
the substance? Are the specific heat capacities of two different materials the same?
2. How does the specific heat capacity of water moderate the climate in a region near a large lake?
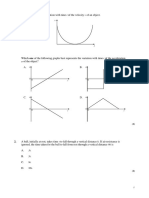
3. The given graph is the specific heat of water as a function of
temperature. Does the specific heat capacity depend on
temperature?
G11-C05-Q02-D: Calculations.
1. How much heat does it take to raise the temperature of 220 g of water from 25 C to 100 C? Specific
heat capacity of water is 4184 J kg1 K1.
2. How much heat must be added to change the temperature of 0.15 kg helium from 30 C to 80 C
without changing the volume? What will happen if the volume changes?
(Specific heat capacity of helium is 5.18 103 J kg1 K1)
You might also like
- “Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4From Everand“Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4No ratings yet
- Gr11 Rev Ch05 02 QnA AnswersDocument3 pagesGr11 Rev Ch05 02 QnA AnswersAidanNo ratings yet
- Gr11 Rev Ch05 01 QnADocument2 pagesGr11 Rev Ch05 01 QnAAidanNo ratings yet
- Lecture Notes On CE HeatDocument44 pagesLecture Notes On CE HeatRichard WongNo ratings yet
- Class X Physics Chapter 11 - Calorimetry Exercise 11 (A)Document16 pagesClass X Physics Chapter 11 - Calorimetry Exercise 11 (A)Isha PatelNo ratings yet
- Gr11 Rev Ch05 03 QnADocument2 pagesGr11 Rev Ch05 03 QnAAidanNo ratings yet
- Heat FOUNDATIONDocument9 pagesHeat FOUNDATIONHarilal K GNo ratings yet
- Thermal PhysicsDocument7 pagesThermal PhysicsMido YoussefNo ratings yet
- Unit 5 ThermodynamicsDocument6 pagesUnit 5 Thermodynamicsමේනුක සූවින්දNo ratings yet
- Topic 3 Thermal Physics - IB PhysicsDocument1 pageTopic 3 Thermal Physics - IB PhysicsFortNite KIDNo ratings yet
- Thermal Equilibrium: TemperatureDocument21 pagesThermal Equilibrium: TemperatureFadlan RoslanNo ratings yet
- Lectures 1 and 2 Temp SHC and ExpansionDocument23 pagesLectures 1 and 2 Temp SHC and ExpansionChrise RajNo ratings yet
- Ch-19 Solids, Liquids and GasesDocument10 pagesCh-19 Solids, Liquids and Gasesananafra861No ratings yet
- Heat and TemperatureDocument6 pagesHeat and TemperatureJavontay StewartNo ratings yet
- PHYSICS21 Heat and TemperatureDocument15 pagesPHYSICS21 Heat and Temperatureapi-3805293100% (1)
- 13 - Thermal Properties of MaterialsDocument11 pages13 - Thermal Properties of MaterialsJsquareNo ratings yet
- Heat CapacityDocument11 pagesHeat Capacitymohammed merkhasNo ratings yet
- Xi Physics - Thermal Properties of MatterDocument14 pagesXi Physics - Thermal Properties of MatteradarshdarasinghNo ratings yet
- Temperature: Heat TransferDocument33 pagesTemperature: Heat TransferDave MendozaNo ratings yet
- Module 7 HeatDocument6 pagesModule 7 Heatmandisamsimango85No ratings yet
- Heat and Temperature NotesDocument2 pagesHeat and Temperature NotesMARISTELA MACARANASNo ratings yet
- Chapter 6Document67 pagesChapter 6dodaNo ratings yet
- CMY 117 - Theme 9 - Thermochemistry (Thermodynamics)Document66 pagesCMY 117 - Theme 9 - Thermochemistry (Thermodynamics)Riyaadh MayetNo ratings yet
- Laboratory: Submitted To: Mabel YaconDocument16 pagesLaboratory: Submitted To: Mabel YaconMacky NoveraNo ratings yet
- 10th CalorimetryDocument35 pages10th CalorimetryKrushnal GadadeNo ratings yet
- Topic+2 +1st+Law+Thermodynamics+1+Aug+2022Document40 pagesTopic+2 +1st+Law+Thermodynamics+1+Aug+2022mpumelaqqNo ratings yet
- Topic 3 Multiple ChoiceDocument8 pagesTopic 3 Multiple ChoiceGajendraNo ratings yet
- Thermal Energy.Document7 pagesThermal Energy.romaehab201912No ratings yet
- Lecture 2Document33 pagesLecture 2ArabellaNo ratings yet
- X MPL PhyDocument111 pagesX MPL PhysugothaaaNo ratings yet
- Science 8 Module 5Document8 pagesScience 8 Module 5Kristel TelmoNo ratings yet
- Form 4 Physics - HeatDocument8 pagesForm 4 Physics - HeatDenisse ChiaNo ratings yet
- Specific Heat of Metals: Experiment # 3Document4 pagesSpecific Heat of Metals: Experiment # 3princess SH IIINo ratings yet
- Heat (Add Science) OkDocument35 pagesHeat (Add Science) OkJaswardi Anwar Bin Md Yaacob� IPGKKBNo ratings yet
- 7.2.1 - Thermal PhysicsDocument22 pages7.2.1 - Thermal Physicsmaha mohNo ratings yet
- Thermal Energy and HeatDocument34 pagesThermal Energy and HeatMarie Ann SeraficaNo ratings yet
- Chapter 9 - Heat and TemperatureDocument7 pagesChapter 9 - Heat and TemperatureLesther James CastroNo ratings yet
- Thermal Physics CoreDocument150 pagesThermal Physics CoreTenisha CastilloNo ratings yet
- CSEC Physics 12 Hour Crash Course Sections B and C Student Version-2Document107 pagesCSEC Physics 12 Hour Crash Course Sections B and C Student Version-2Mrs MendezNo ratings yet
- Thermal Physics - 1Document54 pagesThermal Physics - 1big chungusNo ratings yet
- Physics Lab Experiment 6Document6 pagesPhysics Lab Experiment 6Peter Sam CoNo ratings yet
- 8 Nibqis PKEZpp FDPWDUqDocument24 pages8 Nibqis PKEZpp FDPWDUqmrockzedzNo ratings yet
- Basic Concepts of Thermo With Examples With Solutions Part 2Document20 pagesBasic Concepts of Thermo With Examples With Solutions Part 2arunyogNo ratings yet
- Lesson+6 02+studysheetDocument3 pagesLesson+6 02+studysheetJoyce Ramirez SteelNo ratings yet
- Heat Capacity and Calorimetry Part 1 With Pen 1106 1Document32 pagesHeat Capacity and Calorimetry Part 1 With Pen 1106 1JOHN DAVE MOISES BALDRIASNo ratings yet
- Bab 3 Heat (ICO7)Document4 pagesBab 3 Heat (ICO7)anung edy nugrohoNo ratings yet
- Chapter 6 Heat and TemperatureDocument9 pagesChapter 6 Heat and TemperatureF1040 AleeyaNo ratings yet
- Actividades 2º ESO Bilingüe Heat and TemperatureDocument7 pagesActividades 2º ESO Bilingüe Heat and Temperaturedavidbio_nrNo ratings yet
- IB CHAPTER 3 - Thermal Properties of MatterDocument15 pagesIB CHAPTER 3 - Thermal Properties of MatterAnastasia VergouNo ratings yet
- Thermal Properties and Temperature (Lesson Notes)Document12 pagesThermal Properties and Temperature (Lesson Notes)Hadia AhmadNo ratings yet
- Temperature and Heat Chapter 4 PhyscsDocument7 pagesTemperature and Heat Chapter 4 PhyscsPLEASURENo ratings yet
- Chapter 10Document8 pagesChapter 10aribahamjad8129No ratings yet
- 4 PhyDocument64 pages4 PhymesfinNo ratings yet
- CH 11 Physics Class 11Document10 pagesCH 11 Physics Class 11Khirod Chandra BarikNo ratings yet
- Specific Heat of IronDocument4 pagesSpecific Heat of Ironapi-150547803No ratings yet
- 10.1 Heat CapacitiesDocument2 pages10.1 Heat CapacitiesAz MYNo ratings yet
- 10.1 Heat CapacitiesDocument2 pages10.1 Heat CapacitiesAz MYNo ratings yet
- Chapter 10Document44 pagesChapter 10Jayaletchumi T. Sambantha MoorthyNo ratings yet
- Heat and TemperatureDocument52 pagesHeat and TemperatureAngilyn LumabasNo ratings yet
- CH 11 Physics Class 11Document10 pagesCH 11 Physics Class 11Khirod Chandra BarikNo ratings yet
- GRFP01 Gr11 Ch03 AllDocument22 pagesGRFP01 Gr11 Ch03 AllAidanNo ratings yet
- GRFP01 Gr11 Ch09 ALLDocument37 pagesGRFP01 Gr11 Ch09 ALLAidanNo ratings yet
- GRFP01 Gr11 Ch04 AllDocument22 pagesGRFP01 Gr11 Ch04 AllAidanNo ratings yet
- Gr10 Rev Ch03 03Document1 pageGr10 Rev Ch03 03AidanNo ratings yet
- GRFP01 Gr11 Ch01toCh06Document126 pagesGRFP01 Gr11 Ch01toCh06AidanNo ratings yet
- Functions Assignment3Document7 pagesFunctions Assignment3AidanNo ratings yet
- Gr10 Rev Ch03 02 WADocument3 pagesGr10 Rev Ch03 02 WAAidanNo ratings yet
- The Factor TheoremDocument4 pagesThe Factor TheoremAidanNo ratings yet
- Functions Assignment4Document6 pagesFunctions Assignment4AidanNo ratings yet
- G11 Rev Ch01 01Document1 pageG11 Rev Ch01 01AidanNo ratings yet
- Functions Assignment1Document3 pagesFunctions Assignment1AidanNo ratings yet
- The Remainder TheoremDocument4 pagesThe Remainder TheoremAidanNo ratings yet
- Gr10 Rev Ch04 02 WADocument1 pageGr10 Rev Ch04 02 WAAidanNo ratings yet
- G10 New Ch08 2023 01 Ray AnsDocument3 pagesG10 New Ch08 2023 01 Ray AnsAidanNo ratings yet
- Gr10 Rev Ch03 04Document2 pagesGr10 Rev Ch03 04AidanNo ratings yet
- Gr10 Rev Ch03 03 WADocument2 pagesGr10 Rev Ch03 03 WAAidanNo ratings yet
- Gr10 Rev Ch03 05 WA SQDocument1 pageGr10 Rev Ch03 05 WA SQAidanNo ratings yet
- G10 New Ch06 Test 2023 01 HeatDocument1 pageG10 New Ch06 Test 2023 01 HeatAidanNo ratings yet
- Gr11 Rev Ch03 05 QDocument3 pagesGr11 Rev Ch03 05 QAidanNo ratings yet
- G10 New Ch08 2023 01 RayDocument1 pageG10 New Ch08 2023 01 RayAidanNo ratings yet
- Gr10 Rev Ch03 05Document2 pagesGr10 Rev Ch03 05AidanNo ratings yet
- Gr11 Rev Ch06 01 QnADocument3 pagesGr11 Rev Ch06 01 QnAAidanNo ratings yet
- Gr10 Rev Ch03 05 WADocument3 pagesGr10 Rev Ch03 05 WAAidanNo ratings yet
- GRFP01 - Gr10 - Ch10 - 05 - MAGNETIC PROPERTIES OF IRON AND STEELDocument7 pagesGRFP01 - Gr10 - Ch10 - 05 - MAGNETIC PROPERTIES OF IRON AND STEELAidanNo ratings yet
- Gr10 Rev Ch04 01 WADocument2 pagesGr10 Rev Ch04 01 WAAidanNo ratings yet
- Gr10 Rev Ch05 02 WADocument3 pagesGr10 Rev Ch05 02 WAAidanNo ratings yet
- Gr10 Rev Ch04 03 WADocument1 pageGr10 Rev Ch04 03 WAAidanNo ratings yet
- Gr11 Rev Ch05 04 QnADocument3 pagesGr11 Rev Ch05 04 QnAAidanNo ratings yet
- 11CD108 Plasticity and Metal Forming - First Series Test PART-A (9 X 2 18 Marks) Answer ALL QuestionsDocument1 page11CD108 Plasticity and Metal Forming - First Series Test PART-A (9 X 2 18 Marks) Answer ALL Questionsselva_raj215414No ratings yet
- Lecture 1-2 PDFDocument49 pagesLecture 1-2 PDFASHISH MEENANo ratings yet
- Molecular Chemical Bonding NotesDocument2 pagesMolecular Chemical Bonding NotesMeera KumarNo ratings yet
- Academic Year 2020 - 2021 - ODD Semester PH8151 - Engineering Physics Unit-V Crystal PhysicsDocument10 pagesAcademic Year 2020 - 2021 - ODD Semester PH8151 - Engineering Physics Unit-V Crystal PhysicsBala NandaNo ratings yet
- Photonics Essentials An Introduction With Experiments 2003Document284 pagesPhotonics Essentials An Introduction With Experiments 2003Kajari Chatterjee100% (1)
- Ansys Report 2Document7 pagesAnsys Report 2thilipkumarNo ratings yet
- Snell Manuscript (Keelys Secrets)Document5 pagesSnell Manuscript (Keelys Secrets)Gregg Martin100% (2)
- Classical Field TheoryDocument35 pagesClassical Field TheorySagar JCNo ratings yet
- Slides - Chapter 09Document88 pagesSlides - Chapter 09Mohammad ShawqiNo ratings yet
- MD VI Shaft DesignDocument11 pagesMD VI Shaft DesignShatendra SahuNo ratings yet
- Rotameter Overview PDFDocument8 pagesRotameter Overview PDFSteve Goke AyeniNo ratings yet
- ProblemsDocument32 pagesProblemsjosiedabatosNo ratings yet
- AngularDocument57 pagesAngularThomas Edoche EdocheNo ratings yet
- Compressor MaterialsDocument4 pagesCompressor MaterialsAhmed HassanNo ratings yet
- Crystal Structure and Correlated Properties DGSGDGDocument23 pagesCrystal Structure and Correlated Properties DGSGDGChinar MathurNo ratings yet
- Chemistry ClassDocument13 pagesChemistry ClassbabuNo ratings yet
- Tides, Tidal Mixing, and Internal WavesDocument11 pagesTides, Tidal Mixing, and Internal WavesAkkferiuggneko-nekomncoba Ingintuggndableqclmaeadant DmentmafbntwitNo ratings yet
- Beam Divergence FiberDocument17 pagesBeam Divergence Fiberding2sg3380No ratings yet
- TappersDocument3 pagesTappersJoao CarameloNo ratings yet
- Atomic Physics Solved Examples - AskIITiansDocument3 pagesAtomic Physics Solved Examples - AskIITiansthiripura sundariNo ratings yet
- JurnalDocument96 pagesJurnalDheva NiaNo ratings yet
- Physical Properties of Soil PDFDocument16 pagesPhysical Properties of Soil PDFAshish MittalNo ratings yet
- CHEM10003 - Mock Exam 1Document17 pagesCHEM10003 - Mock Exam 1Sunny XiaNo ratings yet
- Test 21kinematics20151002Document26 pagesTest 21kinematics20151002laguiiniNo ratings yet
- Session I - DC Circuit AnalysisDocument126 pagesSession I - DC Circuit Analysismohan kumarNo ratings yet
- 3 Marks PDFDocument62 pages3 Marks PDFNithin Aadhav.SNo ratings yet
- Metallic BondDocument10 pagesMetallic BondAbhishek NayakNo ratings yet
- NRQCDDocument106 pagesNRQCDMuhammadArshadNo ratings yet
- Convolution: 8.1. Linear Translation Invariant (LTI or LSI) OperatorsDocument9 pagesConvolution: 8.1. Linear Translation Invariant (LTI or LSI) OperatorsAnonymous OOeTGKMAdDNo ratings yet
- Giza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyFrom EverandGiza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyNo ratings yet
- A Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceFrom EverandA Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceRating: 4 out of 5 stars4/5 (51)
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseFrom EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseRating: 3.5 out of 5 stars3.5/5 (69)
- Knocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldFrom EverandKnocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldRating: 3.5 out of 5 stars3.5/5 (64)
- Summary and Interpretation of Reality TransurfingFrom EverandSummary and Interpretation of Reality TransurfingRating: 5 out of 5 stars5/5 (5)
- The End of Everything: (Astrophysically Speaking)From EverandThe End of Everything: (Astrophysically Speaking)Rating: 4.5 out of 5 stars4.5/5 (157)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterFrom EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterRating: 4.5 out of 5 stars4.5/5 (410)
- A Brief History of Time: From the Big Bang to Black HolesFrom EverandA Brief History of Time: From the Big Bang to Black HolesRating: 4 out of 5 stars4/5 (2193)
- The Holographic Universe: The Revolutionary Theory of RealityFrom EverandThe Holographic Universe: The Revolutionary Theory of RealityRating: 4.5 out of 5 stars4.5/5 (77)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeFrom EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeNo ratings yet
- Lost in Math: How Beauty Leads Physics AstrayFrom EverandLost in Math: How Beauty Leads Physics AstrayRating: 4.5 out of 5 stars4.5/5 (125)
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessFrom EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessRating: 4 out of 5 stars4/5 (6)
- Bedeviled: A Shadow History of Demons in ScienceFrom EverandBedeviled: A Shadow History of Demons in ScienceRating: 5 out of 5 stars5/5 (5)
- The Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldFrom EverandThe Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldRating: 4.5 out of 5 stars4.5/5 (54)
- The Beginning of Infinity: Explanations That Transform the WorldFrom EverandThe Beginning of Infinity: Explanations That Transform the WorldRating: 5 out of 5 stars5/5 (60)
- Quantum Physics: What Everyone Needs to KnowFrom EverandQuantum Physics: What Everyone Needs to KnowRating: 4.5 out of 5 stars4.5/5 (49)
- Quantum Physcis for Beginners: An Easy Guide for Discovering the Hidden Side of Reality One Speck at a TimeFrom EverandQuantum Physcis for Beginners: An Easy Guide for Discovering the Hidden Side of Reality One Speck at a TimeNo ratings yet
- Vibration and Frequency: How to Get What You Want in LifeFrom EverandVibration and Frequency: How to Get What You Want in LifeRating: 4.5 out of 5 stars4.5/5 (13)
- Hyperspace: A Scientific Odyssey Through Parallel Universes, Time Warps, and the 10th DimensionFrom EverandHyperspace: A Scientific Odyssey Through Parallel Universes, Time Warps, and the 10th DimensionRating: 4.5 out of 5 stars4.5/5 (3)
- Packing for Mars: The Curious Science of Life in the VoidFrom EverandPacking for Mars: The Curious Science of Life in the VoidRating: 4 out of 5 stars4/5 (1396)
- The Simulated Multiverse: An MIT Computer Scientist Explores Parallel Universes, The Simulation Hypothesis, Quantum Computing and the Mandela EffectFrom EverandThe Simulated Multiverse: An MIT Computer Scientist Explores Parallel Universes, The Simulation Hypothesis, Quantum Computing and the Mandela EffectRating: 4.5 out of 5 stars4.5/5 (20)
- Transform Your Life And Save The World: Through The Dreamed Of Arrival Of The Rehabilitating Biological Explanation Of The Human ConditionFrom EverandTransform Your Life And Save The World: Through The Dreamed Of Arrival Of The Rehabilitating Biological Explanation Of The Human ConditionRating: 5 out of 5 stars5/5 (2)
- Let There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessFrom EverandLet There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessRating: 4.5 out of 5 stars4.5/5 (57)