Professional Documents
Culture Documents
Review CHEM 2
Uploaded by
Michelle SortoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Review CHEM 2
Uploaded by
Michelle SortoCopyright:
Available Formats
IA>
>
✓ Group I>
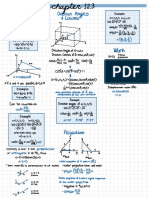
Group 18 Ionic Compounds
' >
Ion
Naming
formation
V
Formula
← ↳ Compound Formation cation
.
anion
cation Anion Lewis structure to tr
IE low
E- A low } IE
high
EA high
g. MgO chargese.
variable
change if name
ides
-
mental
block
}→ variable
Mg 2++25
non
-
• . → to
✓
-
d- block
"
D- block :+2e-→[ :& :] Enervating polyatomic
p block /lower)
-
→ variable ↳ 9 Energy
Lattice
ions
Mg-naring#rgy
"
octet rule <→
Mg "[ :] Mg (g) +0--1 g)
'
Mgocs)
* "
'
is released j
repeating
cry stalling 3-D
netvroic ions
to
held by
[
electrostatic
Trends Approximate attractions
1) high ) ( Coulomb's law
2)
charges
d radius
Using +
→ repulsion
→ small
Ep =
K Q ✗
Qz Epo ceof
d sepr .
231 AJ pm -
I 1 →
hosting
attraction d istbond
You might also like
- Handbook of Chemistry and PhysicsDocument347 pagesHandbook of Chemistry and Physicsdonald1976No ratings yet
- Stoichiometry ModuleDocument8 pagesStoichiometry ModuleKaye NicolasNo ratings yet
- Distillation Column InternalsDocument17 pagesDistillation Column InternalsAhmed Omar AmineNo ratings yet
- Air Pollution Control Technologies: VOC IncineratorsDocument51 pagesAir Pollution Control Technologies: VOC IncineratorsRay CNo ratings yet
- Iso 18254-2015 - ApeoDocument18 pagesIso 18254-2015 - ApeoDoulat RamNo ratings yet
- CB MindmapDocument2 pagesCB Mindmapbob leowNo ratings yet
- Bnie Ia: Prep MDocument10 pagesBnie Ia: Prep MChota CarryNo ratings yet
- Handout-And-HomeworkDocument14 pagesHandout-And-Homeworkvedprakash sumanNo ratings yet
- SilkeDocument8 pagesSilkeChota CarryNo ratings yet
- Va Ut Combiningp#: Elements #Document5 pagesVa Ut Combiningp#: Elements #Muhammad AhmadNo ratings yet
- Catatan Heart FailureDocument3 pagesCatatan Heart FailureYoan hanni YolandaNo ratings yet
- Module 3Document7 pagesModule 3bobby brownNo ratings yet
- Doubts On LogarithmDocument14 pagesDoubts On LogarithmAnshiiiNo ratings yet
- Casting Joints 1 - Karimov Mukhammad-Sodik - 291274Document9 pagesCasting Joints 1 - Karimov Mukhammad-Sodik - 291274Sodik KarimovNo ratings yet
- Catatan Kuliah - Dhita Grace MarcelineDocument1 pageCatatan Kuliah - Dhita Grace MarcelineGrace DhitaNo ratings yet
- UntitledDocument24 pagesUntitledJosieNo ratings yet
- Rayocs: DiscussedDocument4 pagesRayocs: DiscussedMatthew ListroNo ratings yet
- เคมีเพิ่มDocument5 pagesเคมีเพิ่มPavaridNo ratings yet
- 12th Physics - One ShotDocument74 pages12th Physics - One ShotKaranNo ratings yet
- 12th Physics - One ShotDocument62 pages12th Physics - One ShotmaheshNo ratings yet
- É É@ Oearqsoiaf6k: Cs%÷÷ij %Document4 pagesÉ É@ Oearqsoiaf6k: Cs%÷÷ij %Chota CarryNo ratings yet
- PblockDocument13 pagesPblockmandalyuvraj1582No ratings yet
- Coordinating Comp XC12 & A11+Document12 pagesCoordinating Comp XC12 & A11+Krish TomarNo ratings yet
- P Block Class12 Nitesh DevnaniDocument45 pagesP Block Class12 Nitesh DevnaniAdhrita RanaNo ratings yet
- Boee Notes Unit 1 and Unit 2Document110 pagesBoee Notes Unit 1 and Unit 2Vidhi GabaNo ratings yet
- CrystallographyDocument1 pageCrystallographySUNANDAN PANDANo ratings yet
- Salt Analysis JAdvanced Q. 21 To 44 Edited 2Document10 pagesSalt Analysis JAdvanced Q. 21 To 44 Edited 2Sujal KumarNo ratings yet
- StudyDocument7 pagesStudybiacaNo ratings yet
- NOTAS DE AULA UND 1 (Macro I)Document10 pagesNOTAS DE AULA UND 1 (Macro I)Brunno10No ratings yet
- Compusi Halogenati Grile CTDocument5 pagesCompusi Halogenati Grile CTTiberiu CostinNo ratings yet
- Carbon Family Mind MapDocument1 pageCarbon Family Mind Maparyangavli19No ratings yet
- S BlockDocument23 pagesS BlockskyrusttyyNo ratings yet
- Ce133p 2 RCD ComputationDocument16 pagesCe133p 2 RCD ComputationKeroro SeighartNo ratings yet
- Backgro Und PressureDocument2 pagesBackgro Und PressureNader HemayaNo ratings yet
- Procesos IIDocument2 pagesProcesos IIMiranda Escamilla PerezNo ratings yet
- Handnotes Lecture43Document10 pagesHandnotes Lecture43Faheem ShanavasNo ratings yet
- Module-4 1Document14 pagesModule-4 1Prateek TalwarNo ratings yet
- Lesson 15 Notes and ExercisesDocument14 pagesLesson 15 Notes and ExercisesJayson BayogoNo ratings yet
- Response 13 EPC 2Document4 pagesResponse 13 EPC 2Steven HimawanNo ratings yet
- Electrochemical Cell LabDocument3 pagesElectrochemical Cell LabHannah 晗❾No ratings yet
- Apuntes 1º ParcialDocument30 pagesApuntes 1º Parcial6f5d6b5r6tNo ratings yet
- Chemical Kinetics Mind MapDocument1 pageChemical Kinetics Mind MapSamridhi MoudgilNo ratings yet
- BB-6 Sol. Rotational MotionDocument4 pagesBB-6 Sol. Rotational Motionashutoshdwivedi968No ratings yet
- Periodic Properties Live Class-2 Teacher NotesDocument24 pagesPeriodic Properties Live Class-2 Teacher NotesHARSH RAJNo ratings yet
- IB Chemistry Notes (Transition Metals)Document3 pagesIB Chemistry Notes (Transition Metals)hyunjinp0107No ratings yet
- Cardio 2 - X-RayDocument45 pagesCardio 2 - X-RaySaja SaqerNo ratings yet
- 2021-08-18 Is LM FrameworkDocument9 pages2021-08-18 Is LM FrameworkMansi ParmarNo ratings yet
- Untitled Notebook PDFDocument5 pagesUntitled Notebook PDFShehzad QureshiNo ratings yet
- Unit 3 Electrochemistry (Classroom Notes)Document60 pagesUnit 3 Electrochemistry (Classroom Notes)vihas bNo ratings yet
- 2024 04 23 0.42388089858985967Document84 pages2024 04 23 0.42388089858985967RuderNo ratings yet
- Mathemat I KDocument1 pageMathemat I KkerosietmNo ratings yet
- Math 126 NotesDocument10 pagesMath 126 Notesapi-487077331No ratings yet
- 10april ITCM921E01Document10 pages10april ITCM921E01NanaiNo ratings yet
- F Block-ExtractedDocument1 pageF Block-ExtractedKharnikaNo ratings yet
- Modul Tutor THT 2018 PDFDocument1 pageModul Tutor THT 2018 PDFYelsintha SaalinoNo ratings yet
- Brandenburg No. 5 ScoreDocument6 pagesBrandenburg No. 5 ScoreEthan Ruan [CH]No ratings yet
- O.qmm - Xh.iii - Iii.ie//-:::.::::iii: Absorption DesorptionDocument7 pagesO.qmm - Xh.iii - Iii.ie//-:::.::::iii: Absorption DesorptionRamin VisvanichkulNo ratings yet
- 2d ArraysDocument10 pages2d Arraysguptaravii263No ratings yet
- Levenspiel 5 19 MFR KineticsDocument1 pageLevenspiel 5 19 MFR KineticsHarish PrasathNo ratings yet
- Electrochemistry Mind MapDocument2 pagesElectrochemistry Mind MapBhavna BeniwalNo ratings yet
- תרגול גנטיקה נח שמלה סופיDocument3 pagesתרגול גנטיקה נח שמלה סופיNoah ChemlaNo ratings yet
- Ac Bi Stable MagnetDocument1 pageAc Bi Stable Magnetoro plataNo ratings yet
- Carbon Family - BrahmastraDocument35 pagesCarbon Family - BrahmastraStevensonNo ratings yet
- DCM MatrixDocument2 pagesDCM Matrixsonyvioi5No ratings yet
- Notes 6Document1 pageNotes 6Michelle SortoNo ratings yet
- Review CHEMDocument1 pageReview CHEMMichelle SortoNo ratings yet
- Japanese NOTESDocument2 pagesJapanese NOTESMichelle SortoNo ratings yet
- NOTESDocument1 pageNOTESMichelle SortoNo ratings yet
- NOTESDocument1 pageNOTESMichelle SortoNo ratings yet
- PURELAB Classic Spec Sheet LITR38748-05Document2 pagesPURELAB Classic Spec Sheet LITR38748-05Cecilio SantosNo ratings yet
- Sikagard 177 CE PDSDocument5 pagesSikagard 177 CE PDSitskittylimNo ratings yet
- PW .KR B Èku JK (K&Lhesav B V Fof'Kf"V: HKKJRH EkudDocument11 pagesPW .KR B Èku JK (K&Lhesav B V Fof'Kf"V: HKKJRH EkudANMSNo ratings yet
- Confilm - DS 3.07Document2 pagesConfilm - DS 3.07Frederic GagneNo ratings yet
- 01 Anjan Scientific ProfileDocument2 pages01 Anjan Scientific ProfilePushpa RamalingaNo ratings yet
- Week 3 DensityDocument3 pagesWeek 3 DensityannmarieNo ratings yet
- Effects of Prep On CreepDocument11 pagesEffects of Prep On CreepFlo RenceNo ratings yet
- B18pa1 NHN 08 PDFDocument4 pagesB18pa1 NHN 08 PDFMohamed AbdullaNo ratings yet
- Grade 10 Chemistry Week 3 Lesson 2Document2 pagesGrade 10 Chemistry Week 3 Lesson 2Nikoli MajorNo ratings yet
- Potassium Chloride (Powder) : Product Data Sheet (PDS)Document1 pagePotassium Chloride (Powder) : Product Data Sheet (PDS)Mannar1No ratings yet
- BIOL 0052 Biology Ii: LECTURER: Dr. Kherie Rowe FACULTY: Biological and Chemical SciencesDocument24 pagesBIOL 0052 Biology Ii: LECTURER: Dr. Kherie Rowe FACULTY: Biological and Chemical SciencesShandarr BladesNo ratings yet
- OC Sensor Pledia Specifications DocumentDocument2 pagesOC Sensor Pledia Specifications DocumentLucasNo ratings yet
- Physical Chemistry II - Class 17Document24 pagesPhysical Chemistry II - Class 17PARVATHY ANIL - IMS20211No ratings yet
- TribunaloLo Ex#4Document9 pagesTribunaloLo Ex#4Jaylou OpondaNo ratings yet
- 3.15 Revision Guide NMRDocument5 pages3.15 Revision Guide NMRyimiyeh441No ratings yet
- Solutions and SolubilityDocument9 pagesSolutions and SolubilityDarrell W. GarwayNo ratings yet
- Novel Sesquiterpene, 1,2-Epoxyfurano-L0 (15) - Germacren-6-One, The Resin of Engl.Document3 pagesNovel Sesquiterpene, 1,2-Epoxyfurano-L0 (15) - Germacren-6-One, The Resin of Engl.РусланNo ratings yet
- Ceq Apsp eDocument27 pagesCeq Apsp eChess EnjoyerNo ratings yet
- CHM130LL (Experiment 9)Document12 pagesCHM130LL (Experiment 9)sandraNo ratings yet
- High Speed Hard Turning of Aisi S1 (60Wcrv8) Cold Work Tool SteelDocument18 pagesHigh Speed Hard Turning of Aisi S1 (60Wcrv8) Cold Work Tool Steelnovkovic1984No ratings yet
- Environment Degradation of Materials - 1Document27 pagesEnvironment Degradation of Materials - 1NSHIMIYIMANA Jean d'AmourNo ratings yet
- What Are Allosteric Enzymes?Document2 pagesWhat Are Allosteric Enzymes?Anila zafarNo ratings yet
- Test 14Document4 pagesTest 14vidyakumari808940No ratings yet
- Carbon Fiber For A Better WorldDocument3 pagesCarbon Fiber For A Better WorldKhánh Nguyễn NgHNo ratings yet
- Full1 PDFDocument58 pagesFull1 PDFNgan YNo ratings yet