Professional Documents
Culture Documents
Osmosis and Cell Membrane Microbiology Lab Report
Uploaded by
ynaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Osmosis and Cell Membrane Microbiology Lab Report
Uploaded by
ynaCopyright:
Available Formats
LABORATORY REPORT IN
MICROBIOLOGY AND PARASITOLOGY
Jaraba, Khyla M. Exercise 4
BS Psychology 1B April 12, 2023
Associate Professor Carina C. Batol April 13, 2023
OSMOSIS AND THE CELL MEMBRANE
I. Objective
1. To learn the osmosis
2. To observe the effect of salt water on a cell membrane
3. To draw the differences within the two slides
4. To appreciate the osmosis and the cell membrane
II. Materials
1. Microscope
2. Onion epidermis from an onion bulb
3. 2 Microscope slides
4. 2 Coverslips
5. Salt Solution
6. Iodine stain
7. Medicine dropper
8. Water
III. Methodology
1. Peel a thin layer of onion epidermis from the onion bulb.
2. Make two iodine-stained wet mounts by placing a drop of iodine stain on the onion epidermis. Caution:
Iodine is poisonous and causes stain.
3. To one of the wet mount slides, add one drop of the salt solution.
4. To the other wet mount slides, add one drop of water.
5. Observe the slides with the drop of water. Draw what you see under question 1 below.
6. Observe the slide with the drop of salt solution. Draw what you see in question 2 below.
7. In both drawings, label all the following parts; nucleus, cell wall, cytoplasm, and cell membrane.
8. Answer the following questions.
IV. Data and Results
We failed at the exercise on our first attempt. The solutions we required weren't what we had
previously observed. However, we have discovered the purpose of the experiment on our second
attempt. We saw that the cells in the slide with the water drop were definitely bigger and appeared to
be pressing against one another. There is very little, if any, room in between. It does so because water
enters the cell membrane, fills the holes within the cell wall, and causes the cell membrane to become
water-filled. On the slide with a drop of salt solution, however, we observed a variation in cell size. As
the cytoplasm separated from the cell wall, it appeared to contract. Additionally, the slide containing a
drop of salt solution causes the water to evaporate, causing the cells to shrink and become less fully
filled within than the slide containing a drop of water.
V. Drawing
Onion with water Onion with salt solution
VI. Answers to questions
1. Draw three onion cells as they are seen in water under high power.
Please refer my answer to part five drawing
2. Draw three onion cells as seen in salt solution under high power.
Please refers my answer to part five drawing
3. Describe the effect of salt water on the plant cell.
The water is diffusing via osmosis out of the cells, decreasing the quantity of water in their cells and
causing them to be dehydrated.
4. Did the cell membrane allow water to pass out of the cell?
Yes, Water passes through the membrane in a diffusion process called osmosis. During active
transport, energy is expended to assist material movement across the membrane in a direction against
their concentration gradient.
5. (a) Using what you have observed in this activity, tell what you think would happen to plant watered with
salty water.
If you water a plant with salt water, it will eventually die. Additionally, it could cause undesirable
physical changes including yellowing, curling, drooping, and dropping until it eventually dries out and
dies.
(b) Explain why you would expect this to happen.
Since if the salt level in the soil water is too high, water may run back into the soil from the plant roots.
This causes the plant to dehydrate, resulting in a decrease in yield or perhaps mortality
VII. Interpretation of Data and Result
The task is to observe the effect of salt water on a cell membrane. In order to this the materials should
be complete. The onion must be cut into tiny pieces then add water to know what will be the
appearance of this under the high-power objective in the Microscope. After that, in another slide place
small portion of onion with a drop of salt solution then observe in the Microscope under high-power
objective. Lastly, compare the onion with water and salt solution and write what you have been
observed.
VIII. Conclusion
This experiment provides a clear illustration of osmosis. Through a semipermeable barrier in the onion
cells, water molecules are seen to have traveled from the area where they are highly concentrated to the
area where they have a low concentration.
IX. Reference
N/A
You might also like
- Lab 1 - Diffusion and Osmosis Write Up - AP BiologyDocument2 pagesLab 1 - Diffusion and Osmosis Write Up - AP BiologyFVCproductions90% (10)
- O Level Biology Practice Questions And Answers Movement of substancesFrom EverandO Level Biology Practice Questions And Answers Movement of substancesNo ratings yet
- Cec 207 Groundwater ExplorationDocument44 pagesCec 207 Groundwater ExplorationMariam SalamiNo ratings yet
- States of Consciousness, Sleep and DreamsDocument41 pagesStates of Consciousness, Sleep and DreamsynaNo ratings yet
- O Level Biology Practice Questions And Answers Transport In PlantsFrom EverandO Level Biology Practice Questions And Answers Transport In PlantsNo ratings yet
- Lab Report: Cell TransportDocument4 pagesLab Report: Cell Transportmaieunice75% (4)
- O Level Biology Practice Questions And Answers Plant NutritionFrom EverandO Level Biology Practice Questions And Answers Plant NutritionRating: 5 out of 5 stars5/5 (1)
- BIOLOGY Lab Report 2Document4 pagesBIOLOGY Lab Report 2Ziara Jane DimayacyacNo ratings yet
- AP Biology Lab #4 - Diffusion and OsmosisDocument9 pagesAP Biology Lab #4 - Diffusion and OsmosisjcadfNo ratings yet
- Answers in Questions PDFDocument53 pagesAnswers in Questions PDFArasiveluNo ratings yet
- LAB REPORT 1 Wet Mount PreparationDocument7 pagesLAB REPORT 1 Wet Mount PreparationKoreen Bayani100% (1)
- Microbiology Lab Report on OsmosisDocument2 pagesMicrobiology Lab Report on OsmosisynaNo ratings yet
- Onion Cell Membrane Osmosis Lab ReportDocument2 pagesOnion Cell Membrane Osmosis Lab ReportynaNo ratings yet
- LABREP4Document2 pagesLABREP4ynaNo ratings yet
- Osmosis: Diffusion of Water Across a Membrane LabDocument4 pagesOsmosis: Diffusion of Water Across a Membrane LabPei Yu ChenNo ratings yet
- Osmosis in Onion Cell LabDocument2 pagesOsmosis in Onion Cell Lab7ghkw9rsshNo ratings yet
- Diffusion and Osmosis Lab Essential Question: Why Is It Important For Intravenous Fluids Given in A Hospital To HaveDocument6 pagesDiffusion and Osmosis Lab Essential Question: Why Is It Important For Intravenous Fluids Given in A Hospital To HavecrystalNo ratings yet
- Observe Diffusion and Osmosis in Cell SolutionsDocument5 pagesObserve Diffusion and Osmosis in Cell SolutionsDaveDS126No ratings yet
- Scientific Paper On Exercise 5 OsmosisDocument12 pagesScientific Paper On Exercise 5 OsmosisJustin BeltranNo ratings yet
- Lab Report 2Document5 pagesLab Report 2Jessica TysonNo ratings yet
- DiffusionandosmosislabDocument5 pagesDiffusionandosmosislabapi-305603050No ratings yet
- Exercise1 DEMONSTRATION OF OSMOSISDocument5 pagesExercise1 DEMONSTRATION OF OSMOSISKristel Bliss RomanoNo ratings yet
- Biochemistry Lab Report 2Document6 pagesBiochemistry Lab Report 2Ravina SaravananNo ratings yet
- Biology WorksheetDocument4 pagesBiology WorksheetAmmar RizwanNo ratings yet
- Biology Laboratory: School of BiotechnologyDocument8 pagesBiology Laboratory: School of BiotechnologyNgọc Phương Anh NguyễnNo ratings yet
- Ch. 4 - Absorption by Roots - Biology - Class X - ICSE (2019-20) - Unlocked PDFDocument11 pagesCh. 4 - Absorption by Roots - Biology - Class X - ICSE (2019-20) - Unlocked PDFthe lillyNo ratings yet
- Act 3 Gollon, Hanz Chua, ValiantDocument5 pagesAct 3 Gollon, Hanz Chua, Valiants9036282No ratings yet
- LAB 1 (Onion Cells)Document5 pagesLAB 1 (Onion Cells)Rea FrancineNo ratings yet
- Gen Bio - Q2Document20 pagesGen Bio - Q2Mary Angel DimasacatNo ratings yet
- Absorption by RootsDocument6 pagesAbsorption by RootsbirendraNo ratings yet
- Observing Osmosis Flaccid and Turgor in Plant Cells StudentDocument2 pagesObserving Osmosis Flaccid and Turgor in Plant Cells Studentapi-188431847No ratings yet
- IGCSE Answers Chapters 01 05Document7 pagesIGCSE Answers Chapters 01 05María Eugenia MolteniNo ratings yet
- Unit 1 - Self-AssessmentDocument3 pagesUnit 1 - Self-AssessmentCHERRY JILL VILLANUEVANo ratings yet
- Investigation 4 Diffusion and Osmosis PDFDocument13 pagesInvestigation 4 Diffusion and Osmosis PDFAref DahabrahNo ratings yet
- Cells & Tissues 9th BiologyDocument8 pagesCells & Tissues 9th Biologymhussainshigri786No ratings yet
- Pandangan & Sardani - Activity-6-Isolation-Of-MicroorganismsDocument2 pagesPandangan & Sardani - Activity-6-Isolation-Of-MicroorganismsPandangan MatiynNo ratings yet
- Diffusion and Osmosis Lab Report-3Document12 pagesDiffusion and Osmosis Lab Report-3api-502015003No ratings yet
- Biolab Post Lab 1Document13 pagesBiolab Post Lab 1Danely DelfinNo ratings yet
- Diffusion LabDocument10 pagesDiffusion LabDalena HuynhNo ratings yet
- Cell PhysiologyDocument12 pagesCell PhysiologyJaseme OtoyoNo ratings yet
- (Absorption by Roots - Notes) PDFDocument6 pages(Absorption by Roots - Notes) PDFthe lillyNo ratings yet
- Osmosis Demonstration Lab ObjectivesDocument5 pagesOsmosis Demonstration Lab Objectivesapi-308795848No ratings yet
- Cells in Salt and WaterDocument1 pageCells in Salt and WaterSiti OmarNo ratings yet
- Group 2-Excercise 3Document8 pagesGroup 2-Excercise 3James Carbonell Dela PeñaNo ratings yet
- Bio100a Homework 2Document11 pagesBio100a Homework 2lucasaiu9409100% (1)
- 02 Cell Membrane and TransportDocument29 pages02 Cell Membrane and TransportRahmania PamungkasNo ratings yet
- Experiment 2 - PlasmolysisDocument5 pagesExperiment 2 - PlasmolysisR Jay LagdaminNo ratings yet
- Lab Report 1Document5 pagesLab Report 1buiiianna1995No ratings yet
- Osmosis Simulation Through The Cell MembraneDocument12 pagesOsmosis Simulation Through The Cell Membraneapi-345382516No ratings yet
- Lab Report 1Document7 pagesLab Report 1lordyayaNo ratings yet
- Passing Through The Gates of Cells.Document2 pagesPassing Through The Gates of Cells.로델No ratings yet
- Practical Appllication of Osmosis ObservationsDocument4 pagesPractical Appllication of Osmosis ObservationsRichard Christopher BorneaNo ratings yet
- IX Biology Chapter 5 SolutionsDocument3 pagesIX Biology Chapter 5 SolutionsSameep ShahNo ratings yet
- Osmosis in CellsDocument2 pagesOsmosis in CellsColin ParkNo ratings yet
- AbcDocument3 pagesAbcAnnedi ZonNo ratings yet
- Biology 110 Principles of Biology 17 3. Diffusion and OsmosisDocument10 pagesBiology 110 Principles of Biology 17 3. Diffusion and OsmosislauraningsihNo ratings yet
- Osmosis LabDocument2 pagesOsmosis LabCorsea McLaughlinNo ratings yet
- Bio122 - Experiment 2Document11 pagesBio122 - Experiment 2SYLVESTINE JAPOKNo ratings yet
- Section C: Plant Physiology: Plants and FoodDocument8 pagesSection C: Plant Physiology: Plants and FoodMD MotinNo ratings yet
- Biology Manual - STD XDocument23 pagesBiology Manual - STD Xryannemo2008No ratings yet
- BG of The StudyDocument1 pageBG of The StudyynaNo ratings yet
- Reviewer Disaster PDFDocument6 pagesReviewer Disaster PDFynaNo ratings yet
- Reviewer Disaster PDFDocument6 pagesReviewer Disaster PDFynaNo ratings yet
- BerzabalRainierJohn Activity1 OdtDocument1 pageBerzabalRainierJohn Activity1 OdtynaNo ratings yet
- Effects of Open Waste Burning on Air QualityDocument1 pageEffects of Open Waste Burning on Air QualityynaNo ratings yet
- BerzabalRainierJohn Activity1 OdtDocument1 pageBerzabalRainierJohn Activity1 OdtynaNo ratings yet
- ENTREPDocument1 pageENTREPynaNo ratings yet
- Learning by Association: Classical Conditioning: (CR), Which Is The Acquired Response To TheDocument4 pagesLearning by Association: Classical Conditioning: (CR), Which Is The Acquired Response To TheynaNo ratings yet
- 2TSY2223 - GED0001 - TW04-23: Specialized English Program 1Document1 page2TSY2223 - GED0001 - TW04-23: Specialized English Program 1ynaNo ratings yet
- Circle Act No.1Document2 pagesCircle Act No.1ynaNo ratings yet
- Specialized English Program 1Document2 pagesSpecialized English Program 1ynaNo ratings yet
- Microbiology Reviewer - Key ConceptsDocument3 pagesMicrobiology Reviewer - Key ConceptsynaNo ratings yet
- Narrative ReportDocument2 pagesNarrative ReportynaNo ratings yet
- Importance of MicrobiologyDocument3 pagesImportance of MicrobiologyynaNo ratings yet
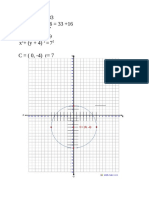
- Solving a quadratic equation: Circle with center (0, -4) and radius 7Document1 pageSolving a quadratic equation: Circle with center (0, -4) and radius 7ynaNo ratings yet
- Circle Act No.2Document1 pageCircle Act No.2ynaNo ratings yet
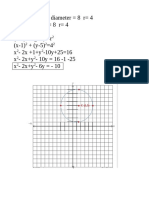
- Find the equation of a circle centered at (0,3Document2 pagesFind the equation of a circle centered at (0,3ynaNo ratings yet
- Laboratory Report in Microbiology and ParasitologyDocument3 pagesLaboratory Report in Microbiology and ParasitologyynaNo ratings yet
- BPSU Endocrine AssignmentDocument6 pagesBPSU Endocrine AssignmentynaNo ratings yet
- GenmathDocument18 pagesGenmathynaNo ratings yet
- Chapter 12 - Urinary SystemDocument50 pagesChapter 12 - Urinary SystemynaNo ratings yet
- Mystery of Thailand's Young King's DeathDocument1 pageMystery of Thailand's Young King's DeathynaNo ratings yet
- Endocrine SystemDocument6 pagesEndocrine SystemynaNo ratings yet
- Discover How Piezoelectric Materials Generate Electricity from MovementDocument9 pagesDiscover How Piezoelectric Materials Generate Electricity from MovementMahmudul HasanNo ratings yet
- Manufactured Substances in Industry: By: Nur Adriana Filzah BT Muhd Khar MunzakarDocument14 pagesManufactured Substances in Industry: By: Nur Adriana Filzah BT Muhd Khar MunzakarHafiy SiddqiNo ratings yet
- Worksheet 4 - Gas Laws and Ideal Gas EquationDocument1 pageWorksheet 4 - Gas Laws and Ideal Gas EquationannmarieNo ratings yet
- The Selection of Soils For Unstabilised Earth Building - A Normative ReviewDocument15 pagesThe Selection of Soils For Unstabilised Earth Building - A Normative ReviewJaqueline ValeNo ratings yet
- Experiment-5 Flakiness Index (FI) Objective Theory: Minimum Mass of Test PortionDocument1 pageExperiment-5 Flakiness Index (FI) Objective Theory: Minimum Mass of Test PortionRefisa JiruNo ratings yet
- ProClin® 950 Preservative For Diagnostic Reagents - Product InformationDocument1 pageProClin® 950 Preservative For Diagnostic Reagents - Product InformationSAFC-GlobalNo ratings yet
- In-And Ga-Based Inorganic Double Perovskites With Direct Bandgaps For Photovoltaic ApplicationDocument6 pagesIn-And Ga-Based Inorganic Double Perovskites With Direct Bandgaps For Photovoltaic Applicationa s m mosabbirNo ratings yet
- Analysis of Mechanical Properties of Natural Fibre Composites by Experimental With FEADocument5 pagesAnalysis of Mechanical Properties of Natural Fibre Composites by Experimental With FEArahul reddyNo ratings yet
- Review UCMDocument3 pagesReview UCMSolo TCNo ratings yet
- European Tracked Micro-Rover For Planetary SurfaceDocument9 pagesEuropean Tracked Micro-Rover For Planetary SurfaceSuryank JoshiNo ratings yet
- Direct Numerical SimulationDocument38 pagesDirect Numerical SimulationddqylxgNo ratings yet
- Factors Influencing Hydrogen Induced CrackingDocument3 pagesFactors Influencing Hydrogen Induced CrackingNapoleon DasNo ratings yet
- The Particulate Nature of MatterDocument15 pagesThe Particulate Nature of Matterabhilasha sharmaNo ratings yet
- Lesson Plan: Lesson: The Mass SpectrometerDocument3 pagesLesson Plan: Lesson: The Mass SpectrometerMarcTnnNo ratings yet
- Module 2Document171 pagesModule 2ddfjfjfds dlijkfdifNo ratings yet
- ACS Periodic Table PDFDocument1 pageACS Periodic Table PDFRyan Christopher MartinezNo ratings yet
- Org Lett 2006 8 2699 - CannabinoidsDocument4 pagesOrg Lett 2006 8 2699 - CannabinoidsFatty BhuwaneeNo ratings yet
- Lab Gak PolymerDocument1 pageLab Gak PolymerRobert LeiNo ratings yet
- Safety Data Sheet: SECTION 1: Identification of The Substance/mixture and of The Company/undertakingDocument8 pagesSafety Data Sheet: SECTION 1: Identification of The Substance/mixture and of The Company/undertakingaloordominicNo ratings yet
- Ce121 Lec5 BuoyancyDocument18 pagesCe121 Lec5 BuoyancyPetForest Ni JohannNo ratings yet
- Photosynthesis and Respiration ExplainedDocument1 pagePhotosynthesis and Respiration ExplainedjazsloanNo ratings yet
- MelamineDocument7 pagesMelamineMohamedNo ratings yet
- Specification For Construction, Testing & Commissioning of Ductile Iron Pipe SystemDocument46 pagesSpecification For Construction, Testing & Commissioning of Ductile Iron Pipe SystemAmro HarasisNo ratings yet
- METALUBE OCG5000 Statement of ConformityDocument3 pagesMETALUBE OCG5000 Statement of ConformityMohammedNo ratings yet
- Lecture 6-Spectroscopic Methods of Analysis - Part 1Document31 pagesLecture 6-Spectroscopic Methods of Analysis - Part 1Leo PisNo ratings yet
- EVS Unit-2 AIR POLLUTIONDocument6 pagesEVS Unit-2 AIR POLLUTIONrohithrock1181No ratings yet
- GEOHIG111 - DCC - Geography Paper 1 - Theory - Mid-Year Exam - June2013 - v5 PDFDocument11 pagesGEOHIG111 - DCC - Geography Paper 1 - Theory - Mid-Year Exam - June2013 - v5 PDFLesediNo ratings yet
- 03-01-21 - JR. STAR CO-SUPER CHAINA - Jee-Main - CTM-19 - SYLLABUS: Sec: JR - CO-SC Date: 03-01-21 Time: 3 Hrs Max. Marks: 300Document18 pages03-01-21 - JR. STAR CO-SUPER CHAINA - Jee-Main - CTM-19 - SYLLABUS: Sec: JR - CO-SC Date: 03-01-21 Time: 3 Hrs Max. Marks: 300Sai SrinivasNo ratings yet
- 01 Hazardous Area Classification FinalDocument20 pages01 Hazardous Area Classification FinalgofechanNo ratings yet