Professional Documents
Culture Documents
Adobe Scan 02 Nov 2022
Uploaded by
Bhavya Somaiya0 ratings0% found this document useful (0 votes)
5 views2 pagesCopyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
5 views2 pagesAdobe Scan 02 Nov 2022
Uploaded by
Bhavya SomaiyaCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
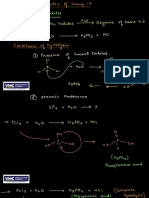
Salt + H2
Nov hem (u » rocess Achin Metal + d
over
ic ) LNDUSTRIAL Bo Mg HCL M, + k
r. u"
2 s not aollecked Steam
C+ 1OOOC A
H + air = explosiv mix Al+H0, ALG0+
Dec dru m qao Laws Coke water gas
5 burns Wth a
+2HCI
10 Cp8 pop sound Steom 2n + Al
p 1o
ret
burns th pale blus flame So fesa, + h
donor.
(1+) woberga Fe
1 H, Ti (Altal)- Oyida
Ampho teic+Alkalk Doulle
H=(1) p a (ralog)- acept (1) *2 NaQH+ O Na CO+ H0 Hudro xide 2al + .
Ammonitaca Za
2Kab Method for P*ps is colleeed +* uprouS Chluide Sol ZnO +
by dsplaumen NaOH No,zno +
H,O
dil HCL
Sol sol + +
OH CuCL 2HO CO Zn (o), KoH
PB NOy CuCL. Co.2H0 n Zno,+ Hho
Araine S CO +NaOH1
2 s only 94 now
As"gJ g So let Pbo NoaZnOa +h
+Nao4
phosphin 7Rea Na,Pbo, + Hho
PH a +Cold
chons [ hoid AlL (OH), + KoH
Me Ho Hdoxid
k,Pbo THo
graulated Ho KOH 3 KOH RALO,+H
uSed
Zn+2Hc Znl,+ t2 acl Na+ H,0
T SUrfa e Na OH
aHea for (dhying agat) Ca AL(OH) NaoH
+O » NaAlO +Ho
in rate absorbs H,o. Act mt Sleom
Ca(OH) Th
af act Mg t Ho hei.OXfda t H Zn/AL/Ph Can react t Aid
Impuvhe Be
boilng
(a alst steam Mgo+H E
44 tH,0
Tnt0 n0 +k
Fe t to maqhe
CU
7Give Reason.
why is (HNO) not preferredin
h_preparation
HNO O+ No+ 0.
Mg +HNO Mg(No+
HNO decomposts to give O, which has a
h'qh affini tor h 3 foms ho
6why is Pb not prcjerrd in th making
dil
Pb+HCL Pb\,+ hT
You might also like
- IB Chemistry HL - ANSWERS - Pearson - Second Edition PDFDocument100 pagesIB Chemistry HL - ANSWERS - Pearson - Second Edition PDFAna Aguilar Garcia67% (49)
- Phenol Mind MapDocument1 pagePhenol Mind MapPriyam PandaNo ratings yet
- Allen Organic QUICK RevisionDocument2 pagesAllen Organic QUICK RevisionChetna Ahlawat100% (2)
- Roadmap Problem - 9Document1 pageRoadmap Problem - 9abhyudaipathwayNo ratings yet
- OQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO DDocument2 pagesOQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO Dmanya9b32100% (1)
- SC21 PDFDocument29 pagesSC21 PDFA Mahmood100% (2)
- Daily Events Check With Horary and Birth Chart: Branches of AstrologyDocument18 pagesDaily Events Check With Horary and Birth Chart: Branches of AstrologySudharshan Srinath50% (2)
- IEC - 60243 Electric Strength of Insulating Materials - Test Methods - Part 2: Additional Requirements For Tests Using Direct VoltageDocument22 pagesIEC - 60243 Electric Strength of Insulating Materials - Test Methods - Part 2: Additional Requirements For Tests Using Direct VoltageAglieglie BrazorNo ratings yet
- Chemistry Paper 1 Topical Unsolved MCQsDocument34 pagesChemistry Paper 1 Topical Unsolved MCQsNobodyNo ratings yet
- Experimental Investigation On Hybrid Fibre Reinforced Concrete by Partial Replacement of MDocument33 pagesExperimental Investigation On Hybrid Fibre Reinforced Concrete by Partial Replacement of MAarthiNo ratings yet
- Roadmap Problem - 1Document1 pageRoadmap Problem - 1Siddharth SharmaNo ratings yet
- Chemistry (Full Test) - Paper 1 - SolutionsDocument6 pagesChemistry (Full Test) - Paper 1 - SolutionsRavi Kiran KoduriNo ratings yet
- Roadmap Problem - 6Document1 pageRoadmap Problem - 6abhyudaipathwayNo ratings yet
- 4 ElectrochemisyryDocument20 pages4 ElectrochemisyryOmkar GhoshNo ratings yet
- Organic Chemistry (2) 70 79Document10 pagesOrganic Chemistry (2) 70 79Mr valorant ipadNo ratings yet
- JEE (Mains) - GTM 12 - 06-01-2020Document11 pagesJEE (Mains) - GTM 12 - 06-01-2020Ravi Kiran KoduriNo ratings yet
- 1-4 Road MapDocument4 pages1-4 Road Mapipsita lahiriNo ratings yet
- Data Booklet FinalDocument2 pagesData Booklet FinalLiqi FengNo ratings yet
- Tabella Pka Molecole OrganicheDocument2 pagesTabella Pka Molecole OrganichePaolo Di PalmaNo ratings yet
- Convertion of Organic CompoundsDocument4 pagesConvertion of Organic CompoundsSanskriti GuptaNo ratings yet
- 10 PrintDocument2 pages10 Printsubin v pNo ratings yet
- Hydro Carbon 3: 29 JanuaryDocument43 pagesHydro Carbon 3: 29 Januarymayank.gupta02005No ratings yet
- Chapter 6: Electrochemistry Example: Ions Attract To The ElectrodeDocument4 pagesChapter 6: Electrochemistry Example: Ions Attract To The ElectrodeAlisa YapNo ratings yet
- AcylChlorides QPDocument26 pagesAcylChlorides QPAnirudh RaoNo ratings yet
- Functional Group Interconversion Scheme PDFDocument1 pageFunctional Group Interconversion Scheme PDFBilal AhmadNo ratings yet
- Homework 1Document7 pagesHomework 1Techno MemerNo ratings yet
- Flow Chart of Organic Reactions: Substitution (+NHDocument1 pageFlow Chart of Organic Reactions: Substitution (+NHAhhhhhhhhhhhNo ratings yet
- Organic Halides Live Class-6 Teacher NotesDocument34 pagesOrganic Halides Live Class-6 Teacher Notesmardarchod 123No ratings yet
- Roadmap Problem - 3Document1 pageRoadmap Problem - 3abhyudaipathwayNo ratings yet
- Synthetic Routes PDFDocument1 pageSynthetic Routes PDFjohn spencerNo ratings yet
- Organic ChemistryDocument1 pageOrganic ChemistryNurul AfiqahNo ratings yet
- NEET/JEE: 2021: Alcohol Phenol EthersDocument2 pagesNEET/JEE: 2021: Alcohol Phenol EthersAmit DeokarNo ratings yet
- 01 Roadmap SolDocument1 page01 Roadmap Solhello helloNo ratings yet
- So Do Phan Ung Hoa Hoc Huu CoDocument6 pagesSo Do Phan Ung Hoa Hoc Huu CoTrường PhúcNo ratings yet
- H.D.A. 2021Document54 pagesH.D.A. 2021Every Time Chemistry [ ETC]No ratings yet
- OCOC-1 Live Class-6 Teacher NotesDocument26 pagesOCOC-1 Live Class-6 Teacher Notesmardarchod 123No ratings yet
- AnionDocument1 pageAnionHarpreet singhNo ratings yet
- Allen Organic Quic RivisionDocument2 pagesAllen Organic Quic Rivisionsaisupreeth0913No ratings yet
- Carbonatotetraamminecobalt (III) Nitrate: H N H N +Document2 pagesCarbonatotetraamminecobalt (III) Nitrate: H N H N +EinsteenNo ratings yet
- Roadmap Problem - 23Document1 pageRoadmap Problem - 23abhyudaipathwayNo ratings yet
- 4102607568703007Document13 pages4102607568703007fragpanthergamingNo ratings yet
- P Block Live Class-2 Teacher NotesDocument55 pagesP Block Live Class-2 Teacher NotesChinmay NagpalNo ratings yet
- Gráficas GeochemDocument5 pagesGráficas Geochemvandrake10No ratings yet
- Aldehyde Ketone and AcidDocument15 pagesAldehyde Ketone and AcidSsNo ratings yet
- Chem CH22Document4 pagesChem CH22OT CANo ratings yet
- Roadmap Problem - 1 PDFDocument1 pageRoadmap Problem - 1 PDFNdjskaNo ratings yet
- Lecture 11, Sulphonation, DiazotizationDocument22 pagesLecture 11, Sulphonation, DiazotizationMALIK ZARYABBABARNo ratings yet
- REACTIVO 2 TermodinamicaDocument5 pagesREACTIVO 2 TermodinamicaCalvin JacobNo ratings yet
- C9 Hydrogen BhiDocument1 pageC9 Hydrogen BhiRunjhunNo ratings yet
- 2 Marathon PDFDocument12 pages2 Marathon PDFsrey sNo ratings yet
- Giao An Day Them Hoa 11Document46 pagesGiao An Day Them Hoa 11265987No ratings yet
- Roadmap Problem - 22Document1 pageRoadmap Problem - 22abhyudaipathwayNo ratings yet
- Problem Set 8Document2 pagesProblem Set 8CARLOS ALBERTO OSORIO MARTINEZNo ratings yet
- X Uv H /PT HX Mno4 H or Oh NH (Alc) Heat NH (Alc) : PCL PCL Socl ZN/HCLDocument1 pageX Uv H /PT HX Mno4 H or Oh NH (Alc) Heat NH (Alc) : PCL PCL Socl ZN/HCLEmily McCullochNo ratings yet
- Organic Reactions Summary SheetDocument2 pagesOrganic Reactions Summary Sheetknprop134No ratings yet
- Completedtitrationnotes 2Document8 pagesCompletedtitrationnotes 2api-336093393No ratings yet
- Organic Chemistry Reactions From MS ChauanDocument3 pagesOrganic Chemistry Reactions From MS ChauanHet GalaNo ratings yet
- RedoxrnDocument5 pagesRedoxrnakshita.singh916No ratings yet
- Aldehyde and KetonesDocument47 pagesAldehyde and KetonesarfanlkoNo ratings yet
- OCOC-1 Live Class-7 Teacher NotesDocument29 pagesOCOC-1 Live Class-7 Teacher Notesmardarchod 123No ratings yet
- O N-NH: Wolf-Kishner ReductionDocument4 pagesO N-NH: Wolf-Kishner ReductiondfghNo ratings yet
- Key Chem TheoryDocument8 pagesKey Chem Theoryalex.holdcroft23No ratings yet
- Carbon Compounds NotesDocument3 pagesCarbon Compounds Notesm-8885756No ratings yet
- Chemistry - Organic Chemistry MechanismsDocument2 pagesChemistry - Organic Chemistry Mechanismshelixate100% (3)
- JK 04 7 Nights 1705560127Document7 pagesJK 04 7 Nights 1705560127Bhavya SomaiyaNo ratings yet
- Highlights of Dubai Abu DhabiDocument9 pagesHighlights of Dubai Abu DhabiBhavya SomaiyaNo ratings yet
- Senior's Special Dubai Abu DhabiDocument10 pagesSenior's Special Dubai Abu DhabiBhavya SomaiyaNo ratings yet
- Grade 10 - Eng Literature - The Patriot-22Document3 pagesGrade 10 - Eng Literature - The Patriot-22Bhavya SomaiyaNo ratings yet
- The Philosophy of Robert Browning April 2013 1598961374 62Document2 pagesThe Philosophy of Robert Browning April 2013 1598961374 62Bhavya SomaiyaNo ratings yet
- Tree AnotationsDocument4 pagesTree AnotationsBhavya SomaiyaNo ratings yet
- Physics 25 - Simple Phenomena of MagntismDocument48 pagesPhysics 25 - Simple Phenomena of MagntismHakim AbbasNo ratings yet
- Solar Cell Literature ReviewDocument6 pagesSolar Cell Literature Reviewaflsktofz100% (1)
- (Course Booklet For PG Section) Handbook of Informationv6Document70 pages(Course Booklet For PG Section) Handbook of Informationv6Pitambar KunduNo ratings yet
- Guía para Seleccionar Columnas HPLCDocument52 pagesGuía para Seleccionar Columnas HPLCDiana Lilibet Sánchez MontesNo ratings yet
- ELECTRICITY NotesDocument62 pagesELECTRICITY Notesmallikammu12No ratings yet
- Cambridge IGCSE™: Physics 0625/42 October/November 2021Document16 pagesCambridge IGCSE™: Physics 0625/42 October/November 2021Manya PunjabiNo ratings yet
- Determine of Morphine and Codeine in Human Urine by Gas Chromatography-Mass SpectrometryDocument7 pagesDetermine of Morphine and Codeine in Human Urine by Gas Chromatography-Mass Spectrometryamaliahriskaika100% (1)
- Discussions On IE Irodov's Problems in General Physics Arihant Books ArihantBooksDocument2 pagesDiscussions On IE Irodov's Problems in General Physics Arihant Books ArihantBooksShootingStarPhotonsNo ratings yet
- C3 Atomic Structure 1Document80 pagesC3 Atomic Structure 1Cassandra mwangiNo ratings yet
- Chapter 1. IntroductionDocument4 pagesChapter 1. IntroductionAmit SawNo ratings yet
- Chemis Chap 4 (f4)Document2 pagesChemis Chap 4 (f4)Kai YuanNo ratings yet
- CL VI Geog First TerminalDocument24 pagesCL VI Geog First TerminalsabirafrinNo ratings yet
- Sure-Shot Questions-Chemistry Class XII: 1markDocument5 pagesSure-Shot Questions-Chemistry Class XII: 1markudit pandyaNo ratings yet
- Silver: This Article Is About The Chemical Element. For The Use of Silver As A Medication, See - For Other Uses, SeeDocument4 pagesSilver: This Article Is About The Chemical Element. For The Use of Silver As A Medication, See - For Other Uses, SeeEllaineNo ratings yet
- Basf MasterGlenium SKY 8614 Tds PDFDocument2 pagesBasf MasterGlenium SKY 8614 Tds PDFSambelteri SelorejoNo ratings yet
- DOE Gasification Program OverviewDocument148 pagesDOE Gasification Program OverviewJohn DalkiaNo ratings yet
- Variant Analysis PPT 27.02.2013Document25 pagesVariant Analysis PPT 27.02.2013UMMID WashimNo ratings yet
- Permainan MolekulDocument5 pagesPermainan MolekulSyarifah R100% (1)
- Eals Notes 1Document7 pagesEals Notes 1Juliana HensonNo ratings yet
- Fluid MechanicsDocument46 pagesFluid MechanicsJaarraa OoNo ratings yet
- Safety Data Sheet Silcone LubricantDocument7 pagesSafety Data Sheet Silcone Lubricanteddy1588No ratings yet
- DKK1413 - Chapter 04-1Document37 pagesDKK1413 - Chapter 04-1Salini ShaNo ratings yet
- Chapter 2 - Tut-1Document7 pagesChapter 2 - Tut-1Anurag PanditNo ratings yet