Professional Documents
Culture Documents
Organic Chemistry
Uploaded by
Nurul AfiqahOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Organic Chemistry
Uploaded by
Nurul AfiqahCopyright:
Available Formats
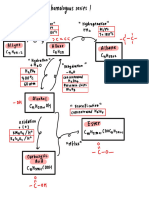
substitution
Alkanes Acids
+Br2, Sunlight or UV ray
co
m
Single-substituted
bu
sti
alkane:
on
C C
2
+O
Cracking or dehydrogenation
Reduction R.A. (eg: H2)
Hydrogenation +H2,, , Ni Catalyst
High temp. press. catalyst
Oxidation O.A. (eg: O2)
Esterification or
CO2 + H2O
condensation
Ester + H2O
, conc. H2SO4
Double-substituted
co
alkane:
mb
2
us
+O
tio
C C
n
Dehydration –H2O, ,
Glucose
Conc. H2SO4
Fermentation solution
Br2 Br2 ition
Alkenes
Hydration +H2O, ,
Alcohols
Add Yeast, 20- 37°C
p.
m tem H3PO4 catalyst
o o
+B r2 r
Polymerisation
Poly(alkane)
Overview of Organic Chemistry~
You might also like
- Oxidation: ReductionDocument7 pagesOxidation: ReductionLeo SukhumvatNo ratings yet
- Photocatalytic Membrane from Recycled Newspaper for Wastewater TreatmentDocument14 pagesPhotocatalytic Membrane from Recycled Newspaper for Wastewater TreatmentWilson UdofiaNo ratings yet
- Breathing-Air-Purifiers Atlas CapcoDocument6 pagesBreathing-Air-Purifiers Atlas CapcoVara PrasadNo ratings yet
- Redox reactions and oxidation numbersDocument2 pagesRedox reactions and oxidation numbersNhiên AnNo ratings yet
- MIND MAP FOR PHENOL DERIVATIVESDocument1 pageMIND MAP FOR PHENOL DERIVATIVESPriyam PandaNo ratings yet
- Adobe Scan 02 Nov 2022Document2 pagesAdobe Scan 02 Nov 2022Bhavya SomaiyaNo ratings yet
- Natalia Gordillo Contreras Analitica Tarea H1Document3 pagesNatalia Gordillo Contreras Analitica Tarea H1NATALIA GORDILLO CONTRERASNo ratings yet
- Chapter 6Document1 pageChapter 6Shaurya JainNo ratings yet
- C9 Hydrogen BhiDocument1 pageC9 Hydrogen BhiRunjhunNo ratings yet
- Organic Chemistry 2Document14 pagesOrganic Chemistry 2Tiên PhạmNo ratings yet
- Organic Chemistry (2) 70 79Document10 pagesOrganic Chemistry (2) 70 79Mr valorant ipadNo ratings yet
- Carbon Compounds NotesDocument3 pagesCarbon Compounds Notesm-8885756No ratings yet
- HW14 ElectrolysisDocument2 pagesHW14 Electrolysiss321012No ratings yet
- Atlas Copco Purificateur - Air BAPDocument8 pagesAtlas Copco Purificateur - Air BAPJimit ShahNo ratings yet
- Everything SummarisedDocument2 pagesEverything SummarisedAryan GovenderNo ratings yet
- Topic 8 and 18 Acid and BasesDocument8 pagesTopic 8 and 18 Acid and BasesChananNo ratings yet
- Carboxylic AcidsDocument1 pageCarboxylic AcidsMd AmanNo ratings yet
- Ammonia Production by Haldor Topsoe Technology: Natural GasDocument1 pageAmmonia Production by Haldor Topsoe Technology: Natural GasUsama JahangirNo ratings yet
- The S - Block Elements Short NotesDocument1 pageThe S - Block Elements Short NotesPinkyNo ratings yet
- The S - Block ElementsDocument1 pageThe S - Block ElementsRunjhunNo ratings yet
- REACTIVO 2 TermodinamicaDocument5 pagesREACTIVO 2 TermodinamicaCalvin JacobNo ratings yet
- Hydrogen - Mind MapDocument1 pageHydrogen - Mind Mapsarthakyedlawar04No ratings yet
- HDA Short NotesDocument4 pagesHDA Short Notesadithaj.2006220No ratings yet
- Acids, Bases, Salts-HWNDocument4 pagesAcids, Bases, Salts-HWNShelin GaziNo ratings yet
- Power-To-Gas - Key Technology For Linking Different Sectors: Heating Systems Refrigeration SystemsDocument6 pagesPower-To-Gas - Key Technology For Linking Different Sectors: Heating Systems Refrigeration Systemsanon_411130333No ratings yet
- Synthetic Routes PDFDocument1 pageSynthetic Routes PDFjohn spencerNo ratings yet
- FROM MULTIPLE METHODS: PREPARATION OF PHENOL FROM ALKENES, DIAZONIUM SALTS, AND CUMENEDocument1 pageFROM MULTIPLE METHODS: PREPARATION OF PHENOL FROM ALKENES, DIAZONIUM SALTS, AND CUMENERonak kadamNo ratings yet
- 20 Carboxylic AcidsDocument10 pages20 Carboxylic AcidsNiki SNo ratings yet
- Complete Water Analysis For Power GenerationDocument12 pagesComplete Water Analysis For Power GenerationRaymund GatocNo ratings yet
- DR Nimmo Part EE A3Document18 pagesDR Nimmo Part EE A3Toks FawibeNo ratings yet
- Organic Chemistry 2023 (2) - 231026 - 083119Document14 pagesOrganic Chemistry 2023 (2) - 231026 - 083119Shankharaj kunduNo ratings yet
- Ror typesDocument1 pageRor typesNawal NCDNo ratings yet
- Index: Experimental Part Results and Discussion Conclusions ReferencesDocument12 pagesIndex: Experimental Part Results and Discussion Conclusions ReferencesJosé Antonio Betancourt CanteraNo ratings yet
- SMDVFV E2Document1 pageSMDVFV E2Ayson Nacino Dela CruzNo ratings yet
- Redox Reactions ExplainedDocument3 pagesRedox Reactions ExplainedHannah 晗❾No ratings yet
- Chapter 6: Electrochemistry Example: Ions Attract To The ElectrodeDocument4 pagesChapter 6: Electrochemistry Example: Ions Attract To The ElectrodeAlisa YapNo ratings yet
- Membuat Struktur Dengan Chemdraw: Nama: Riza Gustina NPM: A1F015009Document1 pageMembuat Struktur Dengan Chemdraw: Nama: Riza Gustina NPM: A1F015009isnaini safitriNo ratings yet
- Aldehyde, Ketones and Carboxylic AcidDocument18 pagesAldehyde, Ketones and Carboxylic AcidSimi Sunil100% (2)
- Pleno Refill EN LowDocument16 pagesPleno Refill EN LowDanijelSeđakNo ratings yet
- Aromatic CompoundDocument15 pagesAromatic Compoundgadhavidhyey5No ratings yet
- Periodic Table of Elements W Oxidation States PubChemDocument1 pagePeriodic Table of Elements W Oxidation States PubChemHuey KaNo ratings yet
- Chem 4.0-4.7 1Document3 pagesChem 4.0-4.7 1henry619leeNo ratings yet
- Top 30 Name ReactionDocument37 pagesTop 30 Name Reactionyadavayush8859No ratings yet
- Gauk 1 Picture1 PDFDocument1 pageGauk 1 Picture1 PDFIshak Ika KovacNo ratings yet
- Natural Production of Polyphenols: Zoo MDocument1 pageNatural Production of Polyphenols: Zoo MIshak Ika KovacNo ratings yet
- Periodic Table of Elements W Atomic Mass PubChemDocument1 pagePeriodic Table of Elements W Atomic Mass PubChemImmaculada AcantoNo ratings yet
- O.qmm - Xh.iii - Iii.ie//-:::.::::iii: Absorption DesorptionDocument7 pagesO.qmm - Xh.iii - Iii.ie//-:::.::::iii: Absorption DesorptionRamin VisvanichkulNo ratings yet
- Narada Lead Carbon Battery TechnologyDocument12 pagesNarada Lead Carbon Battery Technologyambergris200No ratings yet
- LipidsDocument1 pageLipidsMiss MeezNo ratings yet
- 2D C11) Type of ReactionDocument1 page2D C11) Type of Reactionlaurencrowe08No ratings yet
- Biomass For H&P - GasificationDocument42 pagesBiomass For H&P - GasificationAhmad Sederhna AdjaNo ratings yet
- Roadmap Problem - 6Document1 pageRoadmap Problem - 6abhyudaipathwayNo ratings yet
- 5_6228881453233474559Document3 pages5_6228881453233474559Eswara ReddyNo ratings yet
- Redox Reaction Short Notes - Learning Tales 2Document3 pagesRedox Reaction Short Notes - Learning Tales 2Preet KaurNo ratings yet
- เคมีเพิ่มDocument5 pagesเคมีเพิ่มPavaridNo ratings yet
- Garden: WP WPDocument1 pageGarden: WP WPSKYLERNo ratings yet
- Carboxylic Acid 2Document7 pagesCarboxylic Acid 2bisenpallavi80No ratings yet
- Formula SheetDocument2 pagesFormula SheetArcylea FerrerNo ratings yet
- Gambar Atap Bank BNI RUtengDocument5 pagesGambar Atap Bank BNI RUtengrannNo ratings yet
- PB Alumec EnglishDocument12 pagesPB Alumec EnglishByron RodriguezNo ratings yet
- Coreflor: (Non-Metallic Monolithic Surface Hardening Compound)Document2 pagesCoreflor: (Non-Metallic Monolithic Surface Hardening Compound)Ye YintNo ratings yet
- NEET & AIIMS Botany MCQDocument3 pagesNEET & AIIMS Botany MCQDillen JoeNo ratings yet
- Nanomyte SR-100EC: Easy-To-Clean, Scratch-Resistant CoatingDocument1 pageNanomyte SR-100EC: Easy-To-Clean, Scratch-Resistant CoatingLaercio OliveiraNo ratings yet
- Remineralizing Agents: A Comprehensive ReviewDocument4 pagesRemineralizing Agents: A Comprehensive ReviewLanaNo ratings yet
- Lecture Notes Intermolecular ForcesDocument14 pagesLecture Notes Intermolecular ForcesMelvin Pogi138No ratings yet
- Philippines Exporters ListDocument4 pagesPhilippines Exporters ListAnand VermaNo ratings yet
- Quality Control CheckListDocument7 pagesQuality Control CheckListAlpha DekoNo ratings yet
- What Defines An Industrial GasDocument2 pagesWhat Defines An Industrial GasYuri YamirnovNo ratings yet
- Infinity Lubricants MSDS for INF 245 EmulsifierDocument4 pagesInfinity Lubricants MSDS for INF 245 EmulsifierJaleel AhmedNo ratings yet
- Asme Section II A Sa-202 Sa-202mDocument4 pagesAsme Section II A Sa-202 Sa-202mAnonymous GhPzn1xNo ratings yet
- Class 7 - Nutrition in PlantsDocument15 pagesClass 7 - Nutrition in PlantsSMKNo ratings yet
- Ceramic Coating Product Data SheetDocument3 pagesCeramic Coating Product Data SheetANIBALLOPEZVEGANo ratings yet
- CHM 361 - Exp 3Document2 pagesCHM 361 - Exp 3muhamad azlanNo ratings yet
- 3rd Monthly Science Exam ReviewDocument3 pages3rd Monthly Science Exam ReviewKeannoNo ratings yet
- 6464b55599049600185e782d - ## - Some Basic Concept of Chemistry - DPP 10 (Of Lec-15) - Arjuna NEET 2024Document3 pages6464b55599049600185e782d - ## - Some Basic Concept of Chemistry - DPP 10 (Of Lec-15) - Arjuna NEET 2024Lalit SinghNo ratings yet
- Flame and Smoke Retardants in Vinyl ChloDocument27 pagesFlame and Smoke Retardants in Vinyl ChloAroop Ratan SenNo ratings yet
- FIITJEE PHYSICS, CHEMISTRY & MATHEMATICS CPT - 1Document187 pagesFIITJEE PHYSICS, CHEMISTRY & MATHEMATICS CPT - 1Panshulaj PechettyNo ratings yet
- Fire Watcher& Safety WatcherDocument6 pagesFire Watcher& Safety WatcherFarman ShaikhNo ratings yet
- Chemistry: Long Exam 1Document4 pagesChemistry: Long Exam 1Barbara BananaNo ratings yet
- Tailored Textile Chemicals for Specific ApplicationsDocument24 pagesTailored Textile Chemicals for Specific ApplicationsRickgableNo ratings yet
- Introduction To Industrial PharmacyDocument27 pagesIntroduction To Industrial PharmacyDanish Kamal0% (1)
- Chapter 13Document17 pagesChapter 13Oscar Espinosa BonillaNo ratings yet
- Santa Elena Peninsula State University Faculty of Engineering Sciences Petroleum Engineering Casing DesignDocument5 pagesSanta Elena Peninsula State University Faculty of Engineering Sciences Petroleum Engineering Casing DesignFrancisco BurgosNo ratings yet
- AC Quanti Review 2 CC NO AnsDocument5 pagesAC Quanti Review 2 CC NO AnsRoda Gayle RañadaNo ratings yet
- Water 054800Document10 pagesWater 054800anabiedal803No ratings yet
- Class D fire extinguishers for combustible metal firesDocument1 pageClass D fire extinguishers for combustible metal firesJohana IzaguirreNo ratings yet
- Fire Safety & Prevention TipsDocument31 pagesFire Safety & Prevention TipsLakshmi BalaNo ratings yet
- Midterm chm092 2021Document4 pagesMidterm chm092 2021btrsyhmdnNo ratings yet
- Field Joint CoatingDocument7 pagesField Joint Coatingkrishna3794No ratings yet
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Coating and Drying Defects: Troubleshooting Operating ProblemsFrom EverandCoating and Drying Defects: Troubleshooting Operating ProblemsRating: 5 out of 5 stars5/5 (1)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- An Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksFrom EverandAn Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksRating: 5 out of 5 stars5/5 (1)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- Chemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastFrom EverandChemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastNo ratings yet
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Stuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldFrom EverandStuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldRating: 4 out of 5 stars4/5 (289)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- Gas-Liquid And Liquid-Liquid SeparatorsFrom EverandGas-Liquid And Liquid-Liquid SeparatorsRating: 3.5 out of 5 stars3.5/5 (3)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeRating: 4 out of 5 stars4/5 (9)
- Guidelines for Defining Process Safety Competency RequirementsFrom EverandGuidelines for Defining Process Safety Competency RequirementsRating: 3 out of 5 stars3/5 (1)