Professional Documents
Culture Documents
Processes Involving Ideal Gas Formulas
Uploaded by
Yenjie Sy0 ratings0% found this document useful (0 votes)
6 views2 pagesThis document outlines the key thermodynamic processes including isometric, isobaric, isothermal, isentropic, and polytropic processes. It provides the equations that define each process in terms of pressure (P), volume (V), and temperature (T). Specifically, it shows that isometric processes have constant volume, isobaric processes have constant pressure, isothermal processes have constant temperature, isentropic processes involve a polytropic process with k=1, and polytropic processes follow the equation PV^n = constant.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document outlines the key thermodynamic processes including isometric, isobaric, isothermal, isentropic, and polytropic processes. It provides the equations that define each process in terms of pressure (P), volume (V), and temperature (T). Specifically, it shows that isometric processes have constant volume, isobaric processes have constant pressure, isothermal processes have constant temperature, isentropic processes involve a polytropic process with k=1, and polytropic processes follow the equation PV^n = constant.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views2 pagesProcesses Involving Ideal Gas Formulas
Uploaded by
Yenjie SyThis document outlines the key thermodynamic processes including isometric, isobaric, isothermal, isentropic, and polytropic processes. It provides the equations that define each process in terms of pressure (P), volume (V), and temperature (T). Specifically, it shows that isometric processes have constant volume, isobaric processes have constant pressure, isothermal processes have constant temperature, isentropic processes involve a polytropic process with k=1, and polytropic processes follow the equation PV^n = constant.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
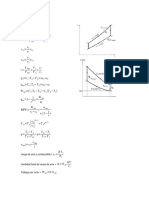
Processes Isometric Isobaric Isothermal Isentropic Polytropic
V1 = V2 p1 = p2 T 1 = T2 PVk = C PVn = C
P, V & T p1 = p2 V1 = V2 p1V1 = p2V2 p1V1k = p2V2k p1V1n = p2V2n
Relations T 1 T2 T 1 T2
T2/T1 = (P2/P1)(k – T2/T1 = (p2/p1)(n –
1)/k 1)/n
T2/T1 = (V1/V2)k – 1 T2/T1 = (V1/V2)n – 1
Nonflow Reversible p1 (V2 – V1) p1V1 ln V2 p2V2 – p1V1 p2V2 – p1V1
Work 0 mR(T2 – T1) V1 1–k 1–n
Wn Irreversible mR(T2 – T1) mR(T2 – T1)
mRT ln p1
∫pdv Q - ∆U
p2 1-k 1-n
Steady flow V1 (p1 – p2) 0 p1V1 ln V2 k{p2V2 – p1V1} n{p2V2 – p1V1}
Work Ws mR(T1 – T2) V1 1-k 1-n
mRT ln p1 - ∆H Q - ∆H
p2
Processes Isometric Isobaric Isothermal Isentropic Polytropic
V1 = V2 p1 = p2 T 1 = T2 pVk = C pVn = C
∆U mcv (T2 – T1) mcv (T2 – T1) 0 mcv (T2 – T1) mcv (T2 – T1)
(U2 – U1)
Heat mcv (T2 – T1) mcp (T2 – T1) p1V1 ln V2 0 mcn (T2 – T1)
Q V1
mRT ln p1
p2
Specific cv cp ∞ 0 cn = cv {(k – n)/1 –n)}
heat (c) (k = C)
∆H mcp (T2 – T1) mcp (T2 – T1) 0 mcp (T2 – T1) mcp (T2 – T1)
(H2 – H1)
∆S mcv ln T2 mcp ln T2 mR ln V2 0 mcn ln T2
(s2 – s1) T1 T1 V1 T1
mR ln p1
p2
You might also like
- P T P T mC ΔT mC ΔT V Δ P mC ΔT mC T T T T P P V V mC ΔT mC ΔT P V P V kWDocument1 pageP T P T mC ΔT mC ΔT V Δ P mC ΔT mC T T T T P P V V mC ΔT mC ΔT P V P V kWJohn Paul BersabeNo ratings yet
- Thermodynamics FormulaDocument9 pagesThermodynamics FormulaJayvie TumangNo ratings yet
- Scroll of Seals 1Document25 pagesScroll of Seals 1Anthony MacalindongNo ratings yet
- ProcessDocument3 pagesProcessSande NasNo ratings yet
- Ideal GasDocument1 pageIdeal GasMike Raphy T. VerdonNo ratings yet
- KinjutsuDocument21 pagesKinjutsuLocus Jhun MichaelNo ratings yet
- D - Thermodynamics 1 - REVIEWDocument1 pageD - Thermodynamics 1 - REVIEWallovidNo ratings yet
- Djj2073 - Thermodynamics: 1. Properties of Pure Substance SteamDocument2 pagesDjj2073 - Thermodynamics: 1. Properties of Pure Substance SteamDkm3a GeraldmosesNo ratings yet
- Inter Nal Energ y (Enthalpy Heat Capacity Q (Under TS) EnthropyDocument3 pagesInter Nal Energ y (Enthalpy Heat Capacity Q (Under TS) EnthropyGood GameNo ratings yet
- ThermodynamicsDocument13 pagesThermodynamicsMonique OrugaNo ratings yet
- Lecture Notes On Compressor 2019 PDFDocument21 pagesLecture Notes On Compressor 2019 PDFJerome MaldaNo ratings yet
- Thermodynamic Processes and DerivationDocument10 pagesThermodynamic Processes and DerivationAbenayaNo ratings yet
- FormulasDocument8 pagesFormulasTecnoLife BaltoNo ratings yet
- Lecture - 7 - First LawDocument9 pagesLecture - 7 - First LawMihai MirceaNo ratings yet
- Formula DJJ20063Document2 pagesFormula DJJ20063NurHiday2010No ratings yet
- Old Thermo Exam Formula SheetDocument1 pageOld Thermo Exam Formula SheetjonahNo ratings yet
- Q W U H S: Isotérmico DT 0 Isobárico DP 0 Isocórico DV 0 Adiabático DQ 0Document2 pagesQ W U H S: Isotérmico DT 0 Isobárico DP 0 Isocórico DV 0 Adiabático DQ 0Aldasaurio SPNo ratings yet
- Thermodynamics Formulae BookletDocument2 pagesThermodynamics Formulae BookletwardeqNo ratings yet
- Adiabatic Ideal GasDocument4 pagesAdiabatic Ideal GasMaria AngelinNo ratings yet
- ThermodynamicsDocument9 pagesThermodynamicssamir boseNo ratings yet
- 1st Law of Thermodynamics-3 PDFDocument9 pages1st Law of Thermodynamics-3 PDFSahmi Abdulqahar NizoriNo ratings yet
- Unit One and ThreeDocument32 pagesUnit One and ThreeGAURAV RATHORENo ratings yet
- FORMULAS XNXNDocument23 pagesFORMULAS XNXNRaymart Layson0% (1)
- Sasi Institute of Technology EnineeringDocument6 pagesSasi Institute of Technology EnineeringHamu NalaNo ratings yet
- Unit 2 First Law-Closed System ProblemsDocument11 pagesUnit 2 First Law-Closed System Problemspiravi66No ratings yet
- Can IdealDocument3 pagesCan IdealHERRERA GAVINO LORIAN GERMANNo ratings yet
- Thermodynamics: The Boundary Work Out of A System (Work Done by System On The Surrounds) Is Defined AsDocument2 pagesThermodynamics: The Boundary Work Out of A System (Work Done by System On The Surrounds) Is Defined AsortizNo ratings yet
- Exam 2 Formula - SheetDocument2 pagesExam 2 Formula - SheetBillyNo ratings yet
- List of Formula MECH 2344Document12 pagesList of Formula MECH 2344hashtagxtahuNo ratings yet
- THERMO1 Formula SheetDocument7 pagesTHERMO1 Formula SheetNyahaha HahahNo ratings yet
- Lecture 7. Vacuum Technology: 7-1. Kinetic Theory of GasesDocument12 pagesLecture 7. Vacuum Technology: 7-1. Kinetic Theory of Gases최종윤No ratings yet
- FORMULASDocument26 pagesFORMULASRaymart LaysonNo ratings yet
- 2.2 The Michelson-Morley Experiment 1Document7 pages2.2 The Michelson-Morley Experiment 1Yuvraj KiskuNo ratings yet
- Termodinamica ch03Document35 pagesTermodinamica ch03Rebeca AlmeidaNo ratings yet
- Final Exam Formu PDFDocument2 pagesFinal Exam Formu PDFJoØrsh Ênrique Tu Xikytø NînîØflowNo ratings yet
- Written Report Thermo Group 10Document8 pagesWritten Report Thermo Group 10Patrick SumalaNo ratings yet
- TermoDocument8 pagesTermoMmtSinotifNo ratings yet
- Formelsammlung - ZustandsänderungenDocument2 pagesFormelsammlung - ZustandsänderungenTrần Nguyên KhôiNo ratings yet
- Kompresible - 1Document11 pagesKompresible - 1isudirmanNo ratings yet
- Midterm Exam - Formula Sheet: Mech 240 - Thermodynamics 1 Winter 2010-2011Document2 pagesMidterm Exam - Formula Sheet: Mech 240 - Thermodynamics 1 Winter 2010-2011Eric LapierreNo ratings yet
- 211 ch3 (Part 2)Document39 pages211 ch3 (Part 2)محمد فاضلNo ratings yet
- Bab 7 Entropi: NugrahaDocument33 pagesBab 7 Entropi: NugrahaGilbert SihombingNo ratings yet
- Summary of ProcessesDocument2 pagesSummary of ProcessesWacko AsahanNo ratings yet
- Lecture14 Wed Oct 11Document3 pagesLecture14 Wed Oct 11Akib ImtihanNo ratings yet
- Formulario Termodinamica 1Document2 pagesFormulario Termodinamica 1Jesus LyroyNo ratings yet
- Formulas For Ideal GasesDocument1 pageFormulas For Ideal GasesAn nguyenhoangNo ratings yet
- Formulário Termodinâmica IDocument2 pagesFormulário Termodinâmica IJoana CostaNo ratings yet
- E Rathakrishnan Gas Dynamics SolutionsDocument216 pagesE Rathakrishnan Gas Dynamics SolutionsVigneshVickey67% (15)
- Power Cycles 1 - 1 PDFDocument6 pagesPower Cycles 1 - 1 PDFclarkmaxNo ratings yet
- Applied Thermodynamics ME250: Submitted ToDocument12 pagesApplied Thermodynamics ME250: Submitted Tomad eye m00dyNo ratings yet
- 6 Processes of Ideal GasDocument14 pages6 Processes of Ideal GasCruz Salise100% (1)
- Physics FormulasDocument2 pagesPhysics FormulasKristine BalansagNo ratings yet
- 1 dH = 1 −q 2 q =a 2hH 3∆ q = = a 2 2g 2gH = ag 2 g H 4 q =λH 5 dH = 1 − λH 6δ H = 1 −λH 7 = 1 1Document3 pages1 dH = 1 −q 2 q =a 2hH 3∆ q = = a 2 2g 2gH = ag 2 g H 4 q =λH 5 dH = 1 − λH 6δ H = 1 −λH 7 = 1 1Bryan ChavezNo ratings yet
- Calculating Cross Sections For Different Processes: Lepton ProductionDocument5 pagesCalculating Cross Sections For Different Processes: Lepton ProductionDaniel GuevaraNo ratings yet
- Full Download Solutions Manual To Accompany Modern Compressible Flow With Historical Perspective 3Rd Edition 9780072424430 PDFDocument43 pagesFull Download Solutions Manual To Accompany Modern Compressible Flow With Historical Perspective 3Rd Edition 9780072424430 PDFdonna.duke560100% (17)
- AC Power AnalysisDocument42 pagesAC Power AnalysisjustinapriochanNo ratings yet
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99From EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99No ratings yet
- Tables of Generalized Airy Functions for the Asymptotic Solution of the Differential Equation: Mathematical Tables SeriesFrom EverandTables of Generalized Airy Functions for the Asymptotic Solution of the Differential Equation: Mathematical Tables SeriesNo ratings yet
- Tables of the Function w (z)- e-z2 ? ex2 dx: Mathematical Tables Series, Vol. 27From EverandTables of the Function w (z)- e-z2 ? ex2 dx: Mathematical Tables Series, Vol. 27No ratings yet
- PhysicsDocument5 pagesPhysicsAyoNo ratings yet
- General Curvilinear Motion (Rectangular Coor)Document18 pagesGeneral Curvilinear Motion (Rectangular Coor)tariqNo ratings yet
- Nissin Di866 Flash User ManualDocument11 pagesNissin Di866 Flash User ManualJulienBarratNo ratings yet
- Determination The Resolving Power of A TelescopeDocument6 pagesDetermination The Resolving Power of A TelescopeAsa mathewNo ratings yet
- Lab Report 11 Slump of Hydraulic Cement ConcreteDocument4 pagesLab Report 11 Slump of Hydraulic Cement ConcreteLeny Agrabio AlaroNo ratings yet
- Flow in Closed Conduits IMDocument18 pagesFlow in Closed Conduits IMJay Wilmer RoqueroNo ratings yet
- Manual T4A3S-B PDFDocument40 pagesManual T4A3S-B PDFCesar Calle JimenezNo ratings yet
- Borchers Rheological Additives: For Waterborne Coating SystemsDocument6 pagesBorchers Rheological Additives: For Waterborne Coating Systemsrogerkid17No ratings yet
- Piping Isometric - 10Document1 pagePiping Isometric - 10CosminMarianNo ratings yet
- Procedure, Magnetic Particle Examination of High Pressure IronDocument7 pagesProcedure, Magnetic Particle Examination of High Pressure IronRalph SanchesNo ratings yet
- Realization of The Zn3+ Oxidation State (d1nr02816b)Document8 pagesRealization of The Zn3+ Oxidation State (d1nr02816b)JohnNo ratings yet
- A Statistical Method For The Determination of Some Properties of The AtomDocument5 pagesA Statistical Method For The Determination of Some Properties of The AtomPedro HenriqueNo ratings yet
- DMV30193-CH 2Document28 pagesDMV30193-CH 2Rothiyah IshakNo ratings yet
- RPMS Engg DBD PP 003Document78 pagesRPMS Engg DBD PP 003RAJKUMARNo ratings yet
- 3 KinematicsDocument37 pages3 KinematicsMaryNo ratings yet
- Soil EngineeringDocument149 pagesSoil EngineeringRenalyn AndradeNo ratings yet
- Schlgl2008 Bulk and SuportsDocument12 pagesSchlgl2008 Bulk and SuportsMaggyBalcazarNo ratings yet
- Linear Guideway (EURO)Document195 pagesLinear Guideway (EURO)PanchoMiyamotoNo ratings yet
- 01.07.01 Flow Diagram 1245 8984Document1 page01.07.01 Flow Diagram 1245 8984周庆卓No ratings yet
- Flow of FluidsDocument2 pagesFlow of FluidsPriyabratNo ratings yet
- Water-Tube Boilers - Part 2: Materials: National Standard of The People'S Republic of ChinaDocument41 pagesWater-Tube Boilers - Part 2: Materials: National Standard of The People'S Republic of ChinaNATTHAPONG BUNSOMPOPNo ratings yet
- Chapter 9 Centroid: Dr. Mahdi Al-FarttoosiDocument32 pagesChapter 9 Centroid: Dr. Mahdi Al-Farttoosiali alkassemNo ratings yet
- Mr. Fawad Tariq, Mr. Muhammad Zeeshan Siddiqui, Ms. Nausheen Naz Mr. Muhammad Fahad Ahmed, Mr. Waqas HussainDocument35 pagesMr. Fawad Tariq, Mr. Muhammad Zeeshan Siddiqui, Ms. Nausheen Naz Mr. Muhammad Fahad Ahmed, Mr. Waqas HussainMahmoud EissaNo ratings yet
- Hardness TestDocument2 pagesHardness TestjorgekarlosprNo ratings yet
- 4Document13 pages4Aruna KumarasiriNo ratings yet
- HPHT Reservoirs - Overview 16-20 - Sep - 2019 PDFDocument44 pagesHPHT Reservoirs - Overview 16-20 - Sep - 2019 PDFVasy Stancu100% (1)
- Efflux Time For A Pipe With Different ExitDocument17 pagesEfflux Time For A Pipe With Different ExitChelley Sharleene PecajasNo ratings yet
- A. Otto Cycle (Gasoline Engine)Document22 pagesA. Otto Cycle (Gasoline Engine)John Manuel BautistaNo ratings yet
- 4 Frontal DisplacementDocument89 pages4 Frontal DisplacementHemant SrivastavaNo ratings yet
- Lecture 03-Design of Doubly Reinforced Beam in FlexureDocument11 pagesLecture 03-Design of Doubly Reinforced Beam in FlexureOmer MehsudNo ratings yet