Professional Documents
Culture Documents
Peka Sains 2
Uploaded by
pkrajenpillaygmailcom0 ratings0% found this document useful (0 votes)
29 views12 pagesThis experiment investigates the heat changes that occur during chemical reactions. The hypothesis is that the reaction between ammonium chloride and water involves heat gain, making it endothermic, while the reaction between sodium hydroxide and water involves heat loss, making it exothermic. The experiment measures the temperature change when ammonium chloride or sodium hydroxide is added to water. The results support the hypothesis, showing the ammonium chloride reaction causes a temperature increase while the sodium hydroxide reaction causes a temperature decrease.
Original Description:
K
Original Title
PEKA SAINS 2
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis experiment investigates the heat changes that occur during chemical reactions. The hypothesis is that the reaction between ammonium chloride and water involves heat gain, making it endothermic, while the reaction between sodium hydroxide and water involves heat loss, making it exothermic. The experiment measures the temperature change when ammonium chloride or sodium hydroxide is added to water. The results support the hypothesis, showing the ammonium chloride reaction causes a temperature increase while the sodium hydroxide reaction causes a temperature decrease.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
29 views12 pagesPeka Sains 2
Uploaded by
pkrajenpillaygmailcomThis experiment investigates the heat changes that occur during chemical reactions. The hypothesis is that the reaction between ammonium chloride and water involves heat gain, making it endothermic, while the reaction between sodium hydroxide and water involves heat loss, making it exothermic. The experiment measures the temperature change when ammonium chloride or sodium hydroxide is added to water. The results support the hypothesis, showing the ammonium chloride reaction causes a temperature increase while the sodium hydroxide reaction causes a temperature decrease.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 12
1 Aim :
To investigate the heat changes
in chemical reactions.
2 Hypothesis :
The reaction between ammonium chloride
and water involves heat gain while the
reaction sodium hydroxide and water
involves heat loss .
3 Variables :
i) Manipulated : Types of reactants
ii) Responding : Change in
temperature readings
iii) Constant : Volume of water
4 Apparatus and materials :
Boiling tubes, spatula, thermometer,
glass rod, sodium hydroxide,
ammonium chloride and distilled water.
5 Procedure :
a) 50 cm3 of distilled water is added into a boiling tube.
b) The initial temperature of the distilled water is
recorded.
c) A spatula full of sodium hydroxide pellets is added
into the boiling tube.
d) The mixture is stirred with a glass rod and the final
temperature is recorded.
e) The change of temperature is calculated.
f) Steps a until e is repeated by replacing sodium
hydroxide pellets with ammonium chloride .
7 Observation :
Initial Final Change in
Reactants temperature temperature temperature
(°C ) (°C ) (°C )
Distilled water
+ Sodium
hydroxide
Distilled water
+ Ammonium
chloride
8 Discussion :
Based on the results obtained from the
reaction between sodium hydroxide and
water, there is an …………….. in the
temperature .
Thus, this reaction is an ……………….
reaction.
Based on the results obtained from the
reaction between ammonium chloride and
water, there is a …………….. in the
temperature .
Thus, this reaction is an ……………….
reaction.
9 Conclusion :
Hypothesis is accepted
(a) The reaction between ammonium chloride and
water has a positive ( + ΔH) heat change.
Therefore, it is an endothermic reaction
(b) The reaction between sodium hydroxide and
water has a negative ( - ΔH) heat change.
Therefore, it is an exothermic reaction.
You might also like
- Heat of solution of ammonium nitrateDocument6 pagesHeat of solution of ammonium nitrateFelix S100% (2)
- Experiment 1 Determination of Enthalpy of Reactions FinalDocument10 pagesExperiment 1 Determination of Enthalpy of Reactions Finalcreate foxesNo ratings yet
- E1 PhychmDocument7 pagesE1 PhychmaenidrisNo ratings yet
- Heat Changes in Chemical Reactions ExperimentDocument9 pagesHeat Changes in Chemical Reactions ExperimentnursyidNo ratings yet
- Sample Evidence 3 TopicDocument2 pagesSample Evidence 3 TopicSalila Mohd SaimNo ratings yet
- Peka - Endothermic and Exothermic ReactionDocument6 pagesPeka - Endothermic and Exothermic ReactionWong Kook LanNo ratings yet
- Experiment-Exothermic ReactionDocument9 pagesExperiment-Exothermic ReactionPau Siew LingNo ratings yet
- EnergeticsDocument9 pagesEnergeticsrichardNo ratings yet
- Heat of NeutralisationDocument5 pagesHeat of NeutralisationNicholas Yaap Wooi YinNo ratings yet
- Energy Chnages in Chemical ReactionsDocument40 pagesEnergy Chnages in Chemical Reactionsmuhammadshadid4No ratings yet
- Chap 9 Thermochemistry-1415 AznitaDocument84 pagesChap 9 Thermochemistry-1415 Aznita黄麒安No ratings yet
- CC Grade 11 Chemistry Energetics CWDocument3 pagesCC Grade 11 Chemistry Energetics CWMaliq MorrisNo ratings yet
- Thermochemistry and Exothermic Endothermic ReactionsDocument4 pagesThermochemistry and Exothermic Endothermic ReactionsJue Hazea GoldshopNo ratings yet
- Lab: Enthalpy of Reaction BackgroundDocument5 pagesLab: Enthalpy of Reaction Backgroundapi-583789375100% (1)
- Sample ChapterDocument7 pagesSample ChapterhugeamountNo ratings yet
- Chapter 8Document84 pagesChapter 8Hafizszul FeyzulNo ratings yet
- ENergetics NeutralizationDocument1 pageENergetics NeutralizationShahiraNo ratings yet
- Exercise.: (A) (Aq) (Aq) (Aq) 2 (L) - 1Document5 pagesExercise.: (A) (Aq) (Aq) (Aq) 2 (L) - 1Pew LingNo ratings yet
- Chem Lab 11Document4 pagesChem Lab 11WHITTINHGAM RAYANNANo ratings yet
- Investigating Temperature Changes - Teacher SheetDocument4 pagesInvestigating Temperature Changes - Teacher SheetNilsp2001No ratings yet
- 3.0 ThermochemistryDocument35 pages3.0 ThermochemistryRoddick BongNo ratings yet
- Heat of Neutralization ReactionDocument5 pagesHeat of Neutralization ReactionbaskhemNo ratings yet
- Very Short Answer Type QuestionsDocument8 pagesVery Short Answer Type QuestionsAnshika YadavNo ratings yet
- Hand Warmer LabDocument12 pagesHand Warmer Labapi-30239979680% (15)
- Investigating Temperature Changes - PracticalDocument4 pagesInvestigating Temperature Changes - PracticalNilsp2001No ratings yet
- Chapter4thermochemistry 150201074346 Conversion Gate02Document38 pagesChapter4thermochemistry 150201074346 Conversion Gate02eric sivaneshNo ratings yet
- Chemical Equations2Document28 pagesChemical Equations2Saleem BashaNo ratings yet
- Experiment 1Document7 pagesExperiment 1Luxemberg Ng100% (4)
- Experiment 2Document6 pagesExperiment 2Syahmi RifqiNo ratings yet
- Thermochemistry Cha4 Form5Document75 pagesThermochemistry Cha4 Form5Azmi IsaacNo ratings yet
- Class 12 Chemistry PracticalDocument21 pagesClass 12 Chemistry PracticalAnand YadavNo ratings yet
- Chemical Reaction and EquationDocument8 pagesChemical Reaction and EquationTr Mazhar Punjabi100% (1)
- HEAT OF NEUTRALIZATION LAB REPORTDocument8 pagesHEAT OF NEUTRALIZATION LAB REPORTBhinitha Chandrasagaran0% (1)
- Basic Principles of Chemistry PracticalsDocument41 pagesBasic Principles of Chemistry PracticalsGodfrey MuchaiNo ratings yet
- Soal Reaksi EksotermDocument8 pagesSoal Reaksi EksotermJack ReacherNo ratings yet
- Lab ReportDocument10 pagesLab ReportFatin Fateha71% (7)
- 9.5 9.6 Review Sheet 1Document8 pages9.5 9.6 Review Sheet 1Soap FabulousNo ratings yet
- EnergeticsDocument11 pagesEnergeticsMuhammadJahangirAlamNo ratings yet
- Chapter 5 - F3Document22 pagesChapter 5 - F3胡欣苓No ratings yet
- Neutralization Reaction Lab ReportDocument4 pagesNeutralization Reaction Lab ReportJohn WangNo ratings yet
- Hess's Law Calorimetry ExperimentDocument6 pagesHess's Law Calorimetry Experimentpufff witchesNo ratings yet
- Example of Exothermic ReactionDocument6 pagesExample of Exothermic ReactionAzriNo ratings yet
- Thermochemistry: APEF - Thermochemistry - Multiple Choice Questions - Answers - Page 1 of 4Document4 pagesThermochemistry: APEF - Thermochemistry - Multiple Choice Questions - Answers - Page 1 of 4BALOGO TRISHA MARIENo ratings yet
- CalorimetroDocument7 pagesCalorimetroYaraNo ratings yet
- Chapter 6-Enthalpy ChangesDocument18 pagesChapter 6-Enthalpy ChangesClarize Soo Hoo0% (1)
- Energy change in chemical reactionsDocument8 pagesEnergy change in chemical reactionsMunshi LazimuzzamanNo ratings yet
- Chapter 2 Thermochemistry Chm271Document32 pagesChapter 2 Thermochemistry Chm271nurul atikaNo ratings yet
- CEAC 104 Son 3 DeneyDocument28 pagesCEAC 104 Son 3 DeneyIbrahim AliNo ratings yet
- Amali Kimia 1 (AutoRecovered)Document14 pagesAmali Kimia 1 (AutoRecovered)SN2-0618 Muhamad Syahmi Rifqi Bin SharimanNo ratings yet
- Experiment 2 Enthalpy of Chemical ReactionsDocument11 pagesExperiment 2 Enthalpy of Chemical ReactionsMirna Carmona100% (3)
- Karen Ann v. BACUS - Activity No.3 - CalorimetryDocument7 pagesKaren Ann v. BACUS - Activity No.3 - CalorimetryKaren Ann V. BACUSNo ratings yet
- Problem Set 2Document5 pagesProblem Set 2UnitedNationsAveNo ratings yet
- CHE1010 Introductory Chemistry TutorialDocument4 pagesCHE1010 Introductory Chemistry TutorialChimuka Onson MapikiNo ratings yet
- Basic Principles of Chemistry PracticalsDocument41 pagesBasic Principles of Chemistry PracticalsMufaro NyamutoraNo ratings yet
- NeutcomDocument12 pagesNeutcomArvin DiNozzoNo ratings yet
- Chemical Reaction and EquationsDocument8 pagesChemical Reaction and Equationsdsarika61No ratings yet
- Exothermic and Endothermic ReactionsDocument3 pagesExothermic and Endothermic ReactionsAhmed KhalilNo ratings yet
- Experiment 1 (Latest)Document16 pagesExperiment 1 (Latest)FadzMieraNo ratings yet
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterFrom EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterRating: 5 out of 5 stars5/5 (1)
- 3 Teresa Science Questions On 22 April 2022Document8 pages3 Teresa Science Questions On 22 April 2022pkrajenpillaygmailcomNo ratings yet
- Colour of Salts and Their IonsDocument1 pageColour of Salts and Their IonspkrajenpillaygmailcomNo ratings yet
- F5 Bio Genetics Part 1Document20 pagesF5 Bio Genetics Part 1pkrajenpillaygmailcomNo ratings yet
- Igcse Questions On ElectrolysisDocument10 pagesIgcse Questions On ElectrolysispkrajenpillaygmailcomNo ratings yet
- Displacement - Time GraphsDocument4 pagesDisplacement - Time GraphspkrajenpillaygmailcomNo ratings yet
- SPM Physics Online Test (Mco)Document2 pagesSPM Physics Online Test (Mco)pkrajenpillaygmailcomNo ratings yet
- f4 Chem p3 Practical 2021 - KSSM Form 4 Apparatus List RequestDocument1 pagef4 Chem p3 Practical 2021 - KSSM Form 4 Apparatus List RequestpkrajenpillaygmailcomNo ratings yet
- School TimetableDocument2 pagesSchool TimetablepkrajenpillaygmailcomNo ratings yet
- Worksheet 1 - Chapter 4 PhysicsDocument7 pagesWorksheet 1 - Chapter 4 PhysicspkrajenpillaygmailcomNo ratings yet
- Latihan Topikal F4 GaramDocument9 pagesLatihan Topikal F4 GaramameermxNo ratings yet
- F4 PBD 1 (Mac) - ADocument2 pagesF4 PBD 1 (Mac) - APkrajen PillaiNo ratings yet
- Cell Organisation in PlantsDocument7 pagesCell Organisation in PlantspkrajenpillaygmailcomNo ratings yet
- Topical Test 3: Chemical Formulae and Equations: Ujian Topikal 3: Formula Dan Persamaan KimiaDocument6 pagesTopical Test 3: Chemical Formulae and Equations: Ujian Topikal 3: Formula Dan Persamaan KimiaameermxNo ratings yet
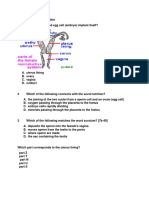
- Questions On ReproductionDocument3 pagesQuestions On ReproductionpkrajenpillaygmailcomNo ratings yet
- Chapter 9 P2Document18 pagesChapter 9 P2faisal850720035887No ratings yet
- Eat ShitDocument1 pageEat ShitpkrajenpillaygmailcomNo ratings yet
- Length & Time 1 QPDocument9 pagesLength & Time 1 QPpkrajenpillaygmailcomNo ratings yet
- Section C Science QuestionDocument8 pagesSection C Science QuestionpkrajenpillaygmailcomNo ratings yet
- Igcse F4 Chemistry TestDocument2 pagesIgcse F4 Chemistry TestpkrajenpillaygmailcomNo ratings yet
- Syllabus Physics 167041-2016-2018-Syllabus PDFDocument45 pagesSyllabus Physics 167041-2016-2018-Syllabus PDFjanpath3834No ratings yet
- 0620 m19 QP 32Document16 pages0620 m19 QP 32pkrajenpillaygmailcomNo ratings yet
- Length & Time (Multiple Choice) QP PDFDocument11 pagesLength & Time (Multiple Choice) QP PDFpkrajenpillaygmailcomNo ratings yet
- Investigate The Physical Properties of Metals and Non MetalsDocument12 pagesInvestigate The Physical Properties of Metals and Non MetalspkrajenpillaygmailcomNo ratings yet
- Chapter 1 F4 ScienceDocument6 pagesChapter 1 F4 SciencepkrajenpillaygmailcomNo ratings yet
- A Acid Base ObjektifDocument4 pagesA Acid Base ObjektifpkrajenpillaygmailcomNo ratings yet
- Investigate The Physical Properties of Metals and Non MetalsDocument3 pagesInvestigate The Physical Properties of Metals and Non MetalspkrajenpillaygmailcomNo ratings yet
- Fizik K1 Melaka SPM 2008 PDFDocument29 pagesFizik K1 Melaka SPM 2008 PDFnizamNo ratings yet
- Investigate The Physical Properties of Metals and Non MetalsDocument3 pagesInvestigate The Physical Properties of Metals and Non MetalspkrajenpillaygmailcomNo ratings yet
- Chem p2 Trial 2019Document15 pagesChem p2 Trial 2019pkrajenpillaygmailcomNo ratings yet