Professional Documents
Culture Documents
5.60 Thermodynamics & Kinetics: Mit Opencourseware
5.60 Thermodynamics & Kinetics: Mit Opencourseware
Uploaded by
Syed Farhan HassanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
5.60 Thermodynamics & Kinetics: Mit Opencourseware
5.60 Thermodynamics & Kinetics: Mit Opencourseware
Uploaded by
Syed Farhan HassanCopyright:
Available Formats
MIT OpenCourseWare http://ocw.mit.
edu
5.60 Thermodynamics & Kinetics
Spring 2008
For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.
5.60 Spring 2008
Lecture #15
page 1
Chemical Equilibrium
Ideal Gases
Question: What is the composition of a reacting mixture of ideal gases? e.g. N2(g, T, p) + 3/2 H2(g, T, p) = NH3(g, T, p)
2 2 3
What are pN , pH , and pNH
at equilibrium?
Lets look at a more general case
A A(g, T, p) + B B(g, T, p) = C C(g, T, p) + D D(g, T, p)
The is are the stoichiometric coefficients. Lets take a mixture of A, B, C, and D with partial pressures
pA = XA p , pB = XA p , pC = XC p , and pD = XD p
Is this mixture in equilibrium? We can answer by finding G if we allow the reaction to proceed further. We know i (T , p ) for an ideal gas in a mixture and we know that G = ni i
i
G ( ) = C C ( g,T , p ) + D D ( g,T , p ) A A ( g,T , p ) + B B ( g,T , p )
5.60 Spring 2008
Lecture #15
page 2
where is an arbitrary small number that allows to let the reaction proceed just a bit. We know that
i ( g,T , p ) = io (T ) + RT ln pi
pi implied 1 bar
where io (T ) is the standard chemical potential of species i at 1 bar and in a pure (not mixed) state.
C D pC p o o o o G ( ) = C C (T ) + D D (T ) A A (T ) + B B (T ) + RT ln A D B pA pB
G = G + RT lnQ
(taking =1)
where and
o o o o G = T ) C C (T ) + D D ( A A (T ) + B B (T )
C D pC p Q = A C is the reaction quotient B pA pB
G is the standard change in free energy for taking pure reactants
into pure products.
o o o G o = Grxn = Hrxn T Srxn
or If
o o G o = Gform ( reactants ) ( products ) Gform
G ( ) < 0
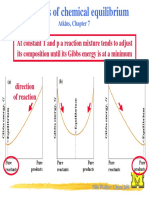
then the reaction will proceed spontaneously to form more products then the backward reaction is spontaneous No spontaneous changes Equilibrium
G ( ) > 0 G ( ) = 0
5.60 Spring 2008
Lecture #15
page 3
o = RT lnQeq At Equilibrium G ( ) = 0 and this implies Grxn
Define
Qeq = K p
the equilibrium constant
D C D C pC pC XC XC K p = A B = p A B = p KX pA pB eq XA XB eq
and thus
o Grxn = RT ln K p , K p = e G
RT
Note from this that K p (T ) is not a function of total pressure p. It is KX = p K p which is KX ( p ,T ) . Recall that all pi values are divided by 1 bar, so Kp and KX are both unitless. ________________________________________________ Example: H2(g) + CO2(g) = H2O(g) + CO(g)
T = 298 K p =1 bar
CO(g) 0
H2(g) Initial # of moles # moles at Eq. a
CO2(g) b
H2O(g) 0
a-x
b-x
Total # moles at Eq. = (a x) + (b x) + 2x = a + b Mole fraction
a x a +b
b x a +b
a +b
a +b
5.60 Spring 2008
Lecture #15
page 4
at Eq.
o Gform (kJ/mol)
-396.6
Kp = e
-228.6
-137.2
and
o rxn
= 28.6 kJ/mol
(8.314 J/K-mol)(298 K )
28,600 kJ/mol
= e 11.54 = 9.7 x 10 6
Kp =
pH O pCO pH pCO
2 2 2
XH OXCO XH XCO
2 2 2
x2 = ( a x ) (b x )
= 9.7 x 10 6
Lets take a = 1 mol and b = 2 mol We need to solve A)
(1 x )(2 x )
x2
Using approximation method: K << 1, so we expect x << 1 also. Assume
1 x 1, 2 x 2
(1 x )(2 x )
x2
x2
2
= 9.7 x 10 6
x 0.0044 mol (indeed << 1)
B)
Exactly:
x2 = K p = 9.7 x 10 6 2 x 3x + 2
x 2 (1 9.7x 10 6 ) + 3x ( 9.7x 10 6 ) 2 ( 9.7x 10 6 ) = 0 x =
3 ( 9.7x 10 6 ) 2 ( 9.7x 10 6 ) 9 ( 9.7x 10 6 ) + 4 (1 9.7x 10 6 ) 2 ( 9.7x 10 6 )
2
2 (1 9.7x 10 6 )
The - sign gives a nonphysical result (negative x value) Take the + sign only x = 0.0044 mol (same)
5.60 Spring 2008
Lecture #15
page 5
Effect of total pressure: example N2O4(g) = 2 NO2(g) Initial mol # # at Eq. n n-x
n x n +x
2
0 2x
2x n +x
Total # moles at Eq. = n x + 2x = n + x
Xis at Eq.
Kp =
pN O
2 pNO
2 p 2XNO
2 4
pXN O
2 4
2x 2 n +x = p 4x =p n2 x 2 n x n + x
Kp = p
4 2 1 2
where = x n is the fraction reacted
Kp = 2 (1 ) 4 p
2
Kp Kp 1 + = 4 p 4p
2
Kp 4p 1 = 2 = Kp 4p 1+ 1 + 4p Kp
4p = 1 + Kp
1 2
If p increases, decreases
Le Chateliers Principle, for pressure: An increase in pressure shifts the equilibrium so as to decrease the total # of moles, reducing the volume. In the example above, increasing p shifts the equilibrium toward the reactants. ---------------
5.60 Spring 2008
Lecture #15
page 6
Another example: 2 NO(g) + O2(g) = 2 NO2(g) Initial mol # # at Eq. 2 2-2x
2 (1 x ) 3x
=
2 p 2XNO
K p = 2.3x 1012 at 298 K
1 1-x
1x 3x
0 2x
2x 3x
Total # moles at Eq. = 2 2x + 1 x + 2x =3-x
Xis at Eq.
Kp =
2 pNO
2 pNO pO
2 p 2XNO pXO
2 p XNO XO
2 XNO
2 1 x (3 x )
(1 x )
K p >> 1 so we expect x 1 3 - x 2
1 2 2 2 x =1 pK p
13
Kp
p (1 x )3
or
(1 x )
pK p
In this case, if p then x as expected from Le Chateliers principle.
You might also like
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Action Plan For Teacher I Iii1Document2 pagesAction Plan For Teacher I Iii1Gesselle Enriquez Salayong - Cambia93% (46)
- Kittel Kroemer Thermal PhysicsDocument53 pagesKittel Kroemer Thermal PhysicsAman-Sharma100% (2)
- Toyota 4a-Fe Engine ReferenceDocument57 pagesToyota 4a-Fe Engine Referencecarmanpy88% (8)
- Cobit 2019 Use Case Handout PDF 08feb19 PDFDocument14 pagesCobit 2019 Use Case Handout PDF 08feb19 PDFАлексей ЦибиногинNo ratings yet
- Equilibrium PDFDocument6 pagesEquilibrium PDFMia Nur AliaNo ratings yet
- 5.60 Thermodynamics & Kinetics: Mit OpencoursewareDocument7 pages5.60 Thermodynamics & Kinetics: Mit OpencoursewarecaptainhassNo ratings yet
- Chemical Equilibrium: Ideal GasesDocument6 pagesChemical Equilibrium: Ideal GasessgybleeNo ratings yet
- Chemical EquilibriumDocument6 pagesChemical Equilibriumlmcristina5No ratings yet
- Chemical Equilibrium: 2.1. Some DefinitionsDocument24 pagesChemical Equilibrium: 2.1. Some DefinitionsNguyễn Quốc HưngNo ratings yet
- Partial Molar PropertiesDocument5 pagesPartial Molar PropertiesRojo JohnNo ratings yet
- Chapter 5Document6 pagesChapter 5Zaki BedraniNo ratings yet
- Thermodynamics - Free PDF DownloadDocument25 pagesThermodynamics - Free PDF Downloadafranazeer16No ratings yet
- Equilibrium in Solution: TPC TPRTC TPRT A TPDocument6 pagesEquilibrium in Solution: TPC TPRTC TPRT A TPsgybleeNo ratings yet
- Final Exam 2015Document6 pagesFinal Exam 2015Kiran JojiNo ratings yet
- 5.60 Thermodynamics & Kinetics: Mit OpencoursewareDocument6 pages5.60 Thermodynamics & Kinetics: Mit OpencoursewareMoneeza AghaNo ratings yet
- Chapter 13 Chemical Reaction EquilibriaDocument35 pagesChapter 13 Chemical Reaction EquilibriaTie Teck HoeNo ratings yet
- CHEMISTRY (CY11003) : Autumn 2021 - 2022Document15 pagesCHEMISTRY (CY11003) : Autumn 2021 - 2022Ashish RanjanNo ratings yet
- T T W Q Q W T T SA W Flux T T W Flux T T J J CM: Carnot Efficiency 1 T TDocument7 pagesT T W Q Q W T T SA W Flux T T W Flux T T J J CM: Carnot Efficiency 1 T TJowel MercadoNo ratings yet
- 16 562ln08Document9 pages16 562ln08sammy wanakaiNo ratings yet
- Second Semester Stage 2Document99 pagesSecond Semester Stage 2Watch DogsNo ratings yet
- Constants and Key Equations in Chemicall ReactionDocument5 pagesConstants and Key Equations in Chemicall ReactionFrancisca BrazNo ratings yet
- Exam3 EquationsheetDocument1 pageExam3 EquationsheetNguyễn Minh AnhNo ratings yet
- Thermodynamics Themochemistry PDFDocument17 pagesThermodynamics Themochemistry PDFAniruddha KawadeNo ratings yet
- Chapter 15: Chemical and Phase Equilibrium: CH O N DCO ECO FO GHO BNDocument0 pagesChapter 15: Chemical and Phase Equilibrium: CH O N DCO ECO FO GHO BNAlex Samuel SilvaNo ratings yet
- ChemistryDocument7 pagesChemistrySankar SasmalNo ratings yet
- 9-Chapter 8-Chemical Reaction Equilibria-27March Online Class-STDNDocument15 pages9-Chapter 8-Chemical Reaction Equilibria-27March Online Class-STDNMahamed HusseinNo ratings yet
- Chapter 13Document46 pagesChapter 13Khloud MadihNo ratings yet
- Chemical Equilibrium FDocument13 pagesChemical Equilibrium FRaju SinghNo ratings yet
- Materiais Temperaturaws CriogênicasDocument53 pagesMateriais Temperaturaws CriogênicasVictor PeterNo ratings yet
- Chemical Thermodynamics 2 Notes On Heterogeneous SystemsDocument5 pagesChemical Thermodynamics 2 Notes On Heterogeneous Systemsian tunanukyeNo ratings yet
- Principles of Chemical EquilibriumDocument6 pagesPrinciples of Chemical EquilibriumJohnS.GallianoNo ratings yet
- 09142710102012introducao A Fisica Estatistica Aula 9Document17 pages09142710102012introducao A Fisica Estatistica Aula 9Rafael BonatoNo ratings yet
- General Chemistry Lecturer-2Document34 pagesGeneral Chemistry Lecturer-2Bảo Long Trần LêNo ratings yet
- Introductory Chemical Engineering Thermodynamics: by J.R. Elliott and C.T. LiraDocument28 pagesIntroductory Chemical Engineering Thermodynamics: by J.R. Elliott and C.T. Lirapenelopezeus39No ratings yet
- Exam 4 - F14-SolutionsDocument10 pagesExam 4 - F14-SolutionsZaimin BhalalaNo ratings yet
- Energy AnsDocument3 pagesEnergy AnskevinamyNo ratings yet
- Topics-Quantum Mechanics 2Document2 pagesTopics-Quantum Mechanics 2BHASWAR MANDALNo ratings yet
- Phase Diagram ExamDocument5 pagesPhase Diagram ExamsaraNo ratings yet
- CMT458 TEST3 Apr 2009 AnsDocument4 pagesCMT458 TEST3 Apr 2009 AnsVanila AisNo ratings yet
- ZZ Chem 1202 Test 1 New Formula SheetDocument5 pagesZZ Chem 1202 Test 1 New Formula Sheetavanit1No ratings yet
- Chapter 5: Equilibrium Composition of Flames: Niranjwan Chettiar 2010213041 ME. Energy Engineering Dr. NatarajanDocument28 pagesChapter 5: Equilibrium Composition of Flames: Niranjwan Chettiar 2010213041 ME. Energy Engineering Dr. NatarajanNiranjwan ChettiarNo ratings yet
- 8 ChemeqDocument6 pages8 ChemeqChristopher Jordan EvoniukNo ratings yet
- Hamiltonian MechanicsDocument19 pagesHamiltonian MechanicsIjaz TalibNo ratings yet
- Lecture 07 - ThermochemistryDocument9 pagesLecture 07 - ThermochemistryHyeon Chang NoNo ratings yet
- Thermo Equations F21Document3 pagesThermo Equations F21BenNo ratings yet
- General Chemistry II Equation SheetDocument2 pagesGeneral Chemistry II Equation Sheetadilb24032000No ratings yet
- Chap 06Document25 pagesChap 06echelon120% (1)
- Kuc: Modified Raoult's Law: ReviewDocument20 pagesKuc: Modified Raoult's Law: ReviewAke TupeslaNo ratings yet
- Thermodynamics of Mixing: N + RT LN P + N + RT LN PDocument12 pagesThermodynamics of Mixing: N + RT LN P + N + RT LN PHuy PhamNo ratings yet
- Pres 2006Document17 pagesPres 2006csreyeroNo ratings yet
- 5.60 Thermodynamics & Kinetics: Mit OpencoursewareDocument7 pages5.60 Thermodynamics & Kinetics: Mit OpencoursewarecaptainhassNo ratings yet
- Compressible Flow PDFDocument210 pagesCompressible Flow PDFRicky JuwonoNo ratings yet
- Chemical EquilibriumDocument142 pagesChemical Equilibriumlaxmikudva47007No ratings yet
- Final Exam Equations, Constants and TablesDocument4 pagesFinal Exam Equations, Constants and Tablessammy alanNo ratings yet
- Numerical Analysis For Real-Time Nonlinear Model Predictive Control (NMPC) of Ethanol Steam ReformersDocument39 pagesNumerical Analysis For Real-Time Nonlinear Model Predictive Control (NMPC) of Ethanol Steam ReformersRobertNo ratings yet
- 4 Properties of GasDocument9 pages4 Properties of GasLuffy01No ratings yet
- Correction of Final January 2022Document3 pagesCorrection of Final January 2022s2ne228No ratings yet
- Fundamentals of Fluid Mechanics Chapter 11 Analysis of Compressible FlowDocument210 pagesFundamentals of Fluid Mechanics Chapter 11 Analysis of Compressible FlowJake OkuyeNo ratings yet
- Analysis of Compressible Flow in FUNDAMENTALS OF FLUID MECHANICSDocument210 pagesAnalysis of Compressible Flow in FUNDAMENTALS OF FLUID MECHANICSAjay kumar100% (1)
- Solution To Statistical Physics Exam: 29th June 2015Document13 pagesSolution To Statistical Physics Exam: 29th June 2015*83*22*No ratings yet
- ThermodynamicsDocument6 pagesThermodynamicsramesh pokhrelNo ratings yet
- CM150-2 Exercise 3 (MODULE 3)Document6 pagesCM150-2 Exercise 3 (MODULE 3)owl lawletNo ratings yet
- d dT J Lq L dx dx: q=J (Π - Π) q=J (Π - Π)Document6 pagesd dT J Lq L dx dx: q=J (Π - Π) q=J (Π - Π)captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: F M F F FDocument5 pages2.57 Nano-to-Macro Transport Processes Fall 2004: F M F F FcaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004 Guest Lecture by Prof. Mildred S. DresselhausDocument10 pages2.57 Nano-to-Macro Transport Processes Fall 2004 Guest Lecture by Prof. Mildred S. DresselhauscaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: I T KXDocument8 pages2.57 Nano-to-Macro Transport Processes Fall 2004: I T KXcaptainhassNo ratings yet
- Q FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004Document5 pagesQ FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- Q FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004Document7 pagesQ FV V: 2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- Lecture 18Document6 pagesLecture 18captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: Nlms 2Document7 pages2.57 Nano-to-Macro Transport Processes Fall 2004: Nlms 2captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document9 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- Lecture 14Document8 pagesLecture 14captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004:) N N N N (NDocument8 pages2.57 Nano-to-Macro Transport Processes Fall 2004:) N N N N (NcaptainhassNo ratings yet
- N F T e U F T F TD D: 2.57 Nano-to-Macro Transport Processes Fall 2004Document6 pagesN F T e U F T F TD D: 2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004 - Lecture 15 Guest Lecture by Prof. DresselhausDocument10 pages2.57 Nano-to-Macro Transport Processes Fall 2004 - Lecture 15 Guest Lecture by Prof. DresselhauscaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: Ae Be CeDocument7 pages2.57 Nano-to-Macro Transport Processes Fall 2004: Ae Be CecaptainhassNo ratings yet
- 2.57 Nano-To-Macro Transport Processes Fall 2004: Ikn A IkxDocument7 pages2.57 Nano-To-Macro Transport Processes Fall 2004: Ikn A IkxcaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: V L KK VKK N VDocument7 pages2.57 Nano-to-Macro Transport Processes Fall 2004: V L KK VKK N VcaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document6 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: E HN MDocument10 pages2.57 Nano-to-Macro Transport Processes Fall 2004: E HN McaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: Ja (j-1) A (j+1) ADocument9 pages2.57 Nano-to-Macro Transport Processes Fall 2004: Ja (j-1) A (j+1) AcaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: A Wigner-Seitz Primitive Unit CellDocument6 pages2.57 Nano-to-Macro Transport Processes Fall 2004: A Wigner-Seitz Primitive Unit CellcaptainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document7 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document7 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004: DT Q K T Q DX Q (W/M K (W/M-K) Is Thermal ConductivityDocument5 pages2.57 Nano-to-Macro Transport Processes Fall 2004: DT Q K T Q DX Q (W/M K (W/M-K) Is Thermal ConductivitycaptainhassNo ratings yet
- L-44 (GDR) (Et) ( (Ee) Nptel)Document15 pagesL-44 (GDR) (Et) ( (Ee) Nptel)yashaswiyellapragadaNo ratings yet
- Problem Solving On D C Machines PDFDocument16 pagesProblem Solving On D C Machines PDFSelvaraj ParamasivanNo ratings yet
- L-05 (GDR) (Et) ( (Ee) Nptel)Document11 pagesL-05 (GDR) (Et) ( (Ee) Nptel)nvnmnitNo ratings yet
- 2.57 Nano-to-Macro Transport Processes Fall 2004Document8 pages2.57 Nano-to-Macro Transport Processes Fall 2004captainhassNo ratings yet
- Three-Phase Induction Motor: Version 2 EE IIT, KharagpurDocument8 pagesThree-Phase Induction Motor: Version 2 EE IIT, KharagpurHarsh PatelNo ratings yet
- L 07 (GDR) (Et) ( (Ee) Nptel)Document15 pagesL 07 (GDR) (Et) ( (Ee) Nptel)Aneurys DuranNo ratings yet
- Photosynthesis Summative ProjectDocument5 pagesPhotosynthesis Summative Projectapi-201420601No ratings yet
- FPGA Based Architecture For Radar's STC, FTC and Gain ModulesDocument7 pagesFPGA Based Architecture For Radar's STC, FTC and Gain Modulesh.moradmand4458No ratings yet
- Discipline and Ideas in The Social Sciences (Diss)Document45 pagesDiscipline and Ideas in The Social Sciences (Diss)Khaite RamosNo ratings yet
- Advanced Immunology and Microbial Science SyllabusDocument4 pagesAdvanced Immunology and Microbial Science SyllabusnasirkgnNo ratings yet
- Notes On Michel FoucaultDocument4 pagesNotes On Michel FoucaultvikNo ratings yet
- BITS Bulletin 2012 PDFDocument616 pagesBITS Bulletin 2012 PDFamit_caliber14No ratings yet
- Juk Mo-6704s 6714S 6716SDocument52 pagesJuk Mo-6704s 6714S 6716SRHoskaNo ratings yet
- LESSON MANUSCRIPT Customs and Tradition of The ServiceDocument20 pagesLESSON MANUSCRIPT Customs and Tradition of The ServiceEden EscaloNo ratings yet
- The Role of Kant's Refutation of IdealismDocument21 pagesThe Role of Kant's Refutation of IdealismKatie SotoNo ratings yet
- Uprtou Assignment HelpDocument4 pagesUprtou Assignment Helpg6aaqevpNo ratings yet
- Intermediate ExcelDocument38 pagesIntermediate ExcelasticksNo ratings yet
- Bibliography 1Document8 pagesBibliography 1Cyril Jane Caanyagan AcutNo ratings yet
- TKDU - Bahasa InggrisDocument9 pagesTKDU - Bahasa InggrisAnna Pryma SarryNo ratings yet
- A Package For Using The BBM Fonts in MathDocument3 pagesA Package For Using The BBM Fonts in Mathgolden gsmNo ratings yet
- Research Methodology - Startup CoffeeDocument18 pagesResearch Methodology - Startup Coffeekidszalor1412100% (1)
- Nanotech SyllabusDocument2 pagesNanotech Syllabusserf007No ratings yet
- Answer SheetDocument4 pagesAnswer SheetĐan KimNo ratings yet
- InaCC-Order Form InaCC06022013Document1 pageInaCC-Order Form InaCC06022013Abduh Aziz BasharahNo ratings yet
- Search Scribd Imaginacion SociologicaDocument12 pagesSearch Scribd Imaginacion SociologicaMarcelo OlivaNo ratings yet
- OfficialresumeDocument2 pagesOfficialresumeapi-322884750No ratings yet
- 1470 001 001 004Document17 pages1470 001 001 004AhmedNo ratings yet
- Final - Healthcare Analytics MDPDocument6 pagesFinal - Healthcare Analytics MDPGvrk AcharyuluNo ratings yet
- Transbase Dokumentation SQL Reference Manual Version 6 8 1Document247 pagesTransbase Dokumentation SQL Reference Manual Version 6 8 1papoiasNo ratings yet
- Rescuing History From Srivijaya The FallDocument55 pagesRescuing History From Srivijaya The FallBabeh AangNo ratings yet
- Eng 161 Final ReviewerDocument15 pagesEng 161 Final Reviewercavi.salvador.auNo ratings yet
- List of Online InternshipsDocument1 pageList of Online InternshipsAshhab Khan100% (1)