0% found this document useful (0 votes)
1K views33 pagesIntroduction to Chromatography Methods
Chromatography is a separation technique that uses differences in how compounds interact with mobile and stationary phases to separate mixtures. The mixture components travel through a supporting medium and separate based on differing rates of movement through the stationary phase coating. Chromatographic methods are classified based on the type of support material (column or planar), nature of the mobile phase (gas, liquid, supercritical fluid), and type of stationary phase. Separation is achieved as analytes interact differently with the phases, moving through the column at different rates and eluting individually to be detected or collected. Band broadening affects column efficiency and resolution of separated components.
Uploaded by
Precious Mae Cuerquis BarbosaCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
1K views33 pagesIntroduction to Chromatography Methods
Chromatography is a separation technique that uses differences in how compounds interact with mobile and stationary phases to separate mixtures. The mixture components travel through a supporting medium and separate based on differing rates of movement through the stationary phase coating. Chromatographic methods are classified based on the type of support material (column or planar), nature of the mobile phase (gas, liquid, supercritical fluid), and type of stationary phase. Separation is achieved as analytes interact differently with the phases, moving through the column at different rates and eluting individually to be detected or collected. Band broadening affects column efficiency and resolution of separated components.
Uploaded by
Precious Mae Cuerquis BarbosaCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
- Basics of Chromatography: Details the basic principles and components of chromatography including mobile and stationary phases.
- Introduction to Chromatography: Presents an overview of chromatography as a separation technique based on compound interaction with two phases.
- Classification of Chromatographic Methods: Describes different classification criteria for chromatographic methods based on support material and stationary phase types.
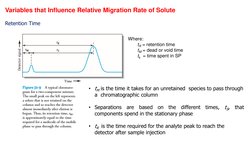
- Elution in Chromatography: Explains the process of elution, defining key terms like eluate and eluent in chromatography.
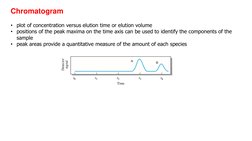
- Chromatogram: Discusses the interpretation of chromatograms, peak maxima and quantitative analysis of peak areas.
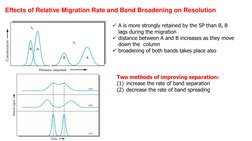
- Effects on Chromatography: Examines effects such as relative migration rates and band broadening on chromatographic resolution.
- Chromatographic Efficiency: Describes retention and selectivity factors, and evaluates column efficiency in chromatographic systems.
- Band Broadening and Column Efficiency: Addresses issues related to band broadening, peak shapes, and how they influence column efficiency.
- Effects of Flow Rate: Explains how the flow rate of the mobile phase affects column efficiency and separation quality.
- Diffusion Terms: Discusses longitudinal diffusion and stationary phase mass-transfer terms affecting chromatography.
- Van Deemter Equation: Details the Van Deemter equation and its role in optimizing chromatographic separations.
- Methods for Reducing Band Broadening: Summarizes techniques for minimizing band broadening and improving chromatographic resolution.
- Optimization Techniques: Covers methods for optimizing chromatographic processes, focusing on resolution and selectivity factor.
- General Elution Problem: Describes challenges in achieving optimal separation, with strategies to address uneven elution profiles.
- Applications of Chromatography: Explores qualitative and quantitative applications of chromatography in analytical chemistry.