Professional Documents
Culture Documents
ΔU + L ΔU = ΔV=: P1 T1 T2 P1
Uploaded by
Lory Pandy0 ratings0% found this document useful (0 votes)
15 views1 pageA gas sample undergoes a polytropic transformation where its volume increases 4 times. The gas initially had a volume of V1 and temperature of T1. During the process, its volume increased to 4V1 while its temperature increased to T2. The heat (Q1,2) received by the gas during this process is calculated to be 82.5RT1.
Original Description:
Original Title
fizica.docx
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentA gas sample undergoes a polytropic transformation where its volume increases 4 times. The gas initially had a volume of V1 and temperature of T1. During the process, its volume increased to 4V1 while its temperature increased to T2. The heat (Q1,2) received by the gas during this process is calculated to be 82.5RT1.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
15 views1 pageΔU + L ΔU = ΔV=: P1 T1 T2 P1
Uploaded by
Lory PandyA gas sample undergoes a polytropic transformation where its volume increases 4 times. The gas initially had a volume of V1 and temperature of T1. During the process, its volume increased to 4V1 while its temperature increased to T2. The heat (Q1,2) received by the gas during this process is calculated to be 82.5RT1.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 1
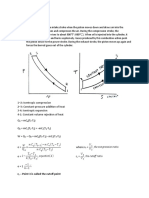
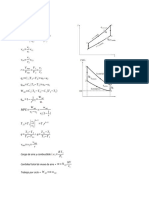
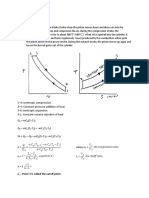
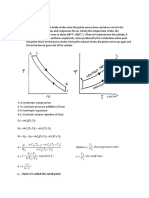
Transformare politropa/ generala
·Un mol de gaz cu Cv = 5R la temperatura T, efectueaza o
transformare politropa in care isi mareste de 4 ori V (a-constanta) Ce
caldura primeste gazul?
Cv=5R Q1,2 = ΔU1,2 + L1,2
µ=1 mol ΔU1,2 = µ · Cv · ΔV = Cv (T2 – T1)
P1 T1 P1 1
T=av2 P1V1 = µR T1 = > P1V1 = µR T1 => 4 P 1 T 2 4 P 2 16 =>
= = =
V=4V1 P2V2 = µR T2 P24V1 = µR T2
Q1,2 = ? => P1 · 4 = P2
T = av2
T1 av 1 2 1
T = av12 => = =
T 2 a 12 v 12 16 => T1 · 16 = T2
T1 = av22 = a · 16v12
(P 1+ P2)(V 2−V 1) 5 P 1 ·3 V 1 5P1V 1 15 µRT 1
L1,2 2
= 2 = 2 = 2
15 µRT 1 15· 1 · RT 1 15 0 RT 1+1 5 RT 1 165 RT 1
Q1,2 = 5 · R ·15T1 + 2 = 75RT1 + 2 = 2 = 2
=82,5 RT1
Suci Lorena
Clasa a X a C
You might also like
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Formulario Termodinamica 1Document2 pagesFormulario Termodinamica 1Jesus LyroyNo ratings yet
- Problem Solving Tips ThermodynamicsDocument5 pagesProblem Solving Tips ThermodynamicsJose FabianNo ratings yet
- Power Cycles 1 - 1 PDFDocument6 pagesPower Cycles 1 - 1 PDFclarkmaxNo ratings yet
- CLS JEEAD-19-20 XI Phy Target-5 Level-2 Chapter-11 PDFDocument28 pagesCLS JEEAD-19-20 XI Phy Target-5 Level-2 Chapter-11 PDFVISHVESH GOYAL100% (1)
- Termodinamica ProblemDocument6 pagesTermodinamica ProblemJuandearco JuanarcoNo ratings yet
- Unit 2 First Law-Closed System ProblemsDocument11 pagesUnit 2 First Law-Closed System Problemspiravi66No ratings yet
- ThermodynamicsDocument13 pagesThermodynamicsMonique OrugaNo ratings yet
- Processes Involving Ideal Gas FormulasDocument2 pagesProcesses Involving Ideal Gas FormulasYenjie SyNo ratings yet
- Assignment 2 Cycles 2020 PDFDocument3 pagesAssignment 2 Cycles 2020 PDFMasa NabaNo ratings yet
- Thermo Cycles PDFDocument29 pagesThermo Cycles PDFWaseem Abbas AttariNo ratings yet
- Bab 7 Entropi: NugrahaDocument33 pagesBab 7 Entropi: NugrahaGilbert SihombingNo ratings yet
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocument5 pagesDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNo ratings yet
- Kmme Te101-3Document6 pagesKmme Te101-3ayla sözenNo ratings yet
- Kompresible - 1Document11 pagesKompresible - 1isudirmanNo ratings yet
- FormulasDocument8 pagesFormulasTecnoLife BaltoNo ratings yet
- Physics - Solution Set For Homework Book Chapter - 15 KTG & ThermodynamicsDocument4 pagesPhysics - Solution Set For Homework Book Chapter - 15 KTG & ThermodynamicsKishorNo ratings yet
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocument7 pagesDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNo ratings yet
- Old Thermo Exam Formula SheetDocument1 pageOld Thermo Exam Formula SheetjonahNo ratings yet
- Principle of TurbomachineryDocument159 pagesPrinciple of TurbomachineryWalid MohammedNo ratings yet
- Applied Thermodynamics ME250: Submitted ToDocument12 pagesApplied Thermodynamics ME250: Submitted Tomad eye m00dyNo ratings yet
- Diesel Cycle: R - Point 3 Is Called The Cutoff PointDocument8 pagesDiesel Cycle: R - Point 3 Is Called The Cutoff PointJethro Briza GaneloNo ratings yet
- Task 1 (L02: P2.1) Kelvin Plank Statement: Efficiency Efficiency (1 Qout QinDocument16 pagesTask 1 (L02: P2.1) Kelvin Plank Statement: Efficiency Efficiency (1 Qout Qinedd bazNo ratings yet
- Equation Thermal Diesel CycleDocument2 pagesEquation Thermal Diesel CycleZakyKikyNo ratings yet
- Thermo Cycles 2Document16 pagesThermo Cycles 2cobalt boronNo ratings yet
- Gas Turbines Unit 3: Gas Turbines and Jet Propulsion: Classification of Gas Turbines, Analysis of Open Cycle GasDocument39 pagesGas Turbines Unit 3: Gas Turbines and Jet Propulsion: Classification of Gas Turbines, Analysis of Open Cycle GasJoseph MwansaNo ratings yet
- Mid-Term Exam #1: Solution by Eric CochranDocument7 pagesMid-Term Exam #1: Solution by Eric Cochranhlclark4768No ratings yet
- 211 ch3 (Part 2)Document39 pages211 ch3 (Part 2)محمد فاضلNo ratings yet
- E Rathakrishnan Gas Dynamics SolutionsDocument216 pagesE Rathakrishnan Gas Dynamics SolutionsVigneshVickey67% (15)
- Gas LawsDocument2 pagesGas Lawskimtaemin1997.stageNo ratings yet
- Marine Diesel (WEEK - 3) Thermodynamics: Jurusan Teknik Sistem Perkapalan ITS SurabayaDocument38 pagesMarine Diesel (WEEK - 3) Thermodynamics: Jurusan Teknik Sistem Perkapalan ITS SurabayafaridNo ratings yet
- Term Odin A MicaDocument10 pagesTerm Odin A MicaFelipe De Lima RomeroNo ratings yet
- FORMULA3Document1 pageFORMULA3agus98No ratings yet
- استنتاج2Document30 pagesاستنتاج2mohamed orifNo ratings yet
- ρ dV C dt ρ F W: For liquid phaseDocument3 pagesρ dV C dt ρ F W: For liquid phaseSelly The SpiceNo ratings yet
- Q W U H S: Isotérmico DT 0 Isobárico DP 0 Isocórico DV 0 Adiabático DQ 0Document2 pagesQ W U H S: Isotérmico DT 0 Isobárico DP 0 Isocórico DV 0 Adiabático DQ 0Aldasaurio SPNo ratings yet
- Prelim Ito Na PreDocument8 pagesPrelim Ito Na PreJethro Briza GaneloNo ratings yet
- Equations (Chapter 5) : For All Closed SystemsDocument2 pagesEquations (Chapter 5) : For All Closed SystemsElzNo ratings yet
- 02 2ndlaw ExessolsDocument14 pages02 2ndlaw Exessolsblanca.pegueraNo ratings yet
- Thermodynamic Processes and DerivationDocument10 pagesThermodynamic Processes and DerivationAbenayaNo ratings yet
- Quiz 1 - MA SolutionsDocument6 pagesQuiz 1 - MA SolutionsabullaNo ratings yet
- Theoretical CyclesDocument49 pagesTheoretical CyclesMariaEzzaSyUyNo ratings yet
- Scroll of Seals 1Document25 pagesScroll of Seals 1Anthony MacalindongNo ratings yet
- Control Valve SizingDocument4 pagesControl Valve SizingAmolNo ratings yet
- Physics 112 Formulae IIIDocument3 pagesPhysics 112 Formulae IIISaied RajehaNo ratings yet
- Tabla 1.1.2.3Document1 pageTabla 1.1.2.3Michel TorresNo ratings yet
- Wa Ter F, T Water Vapour Produce D: Assumptions: No Spatial Variation Incompressible FluidDocument4 pagesWa Ter F, T Water Vapour Produce D: Assumptions: No Spatial Variation Incompressible Fluidcarleston thurgoodNo ratings yet
- Gasturbine 1 160120155226Document52 pagesGasturbine 1 160120155226Muhammad Afif NaufalNo ratings yet
- Che 254 (Midsem)Document6 pagesChe 254 (Midsem)obumaradonaNo ratings yet
- Formulas and Constants - Stp2022Document1 pageFormulas and Constants - Stp2022dayraNo ratings yet
- Adiabatic Ideal GasDocument4 pagesAdiabatic Ideal GasMaria AngelinNo ratings yet
- 1 - Introduction To Phase EquilibriumDocument42 pages1 - Introduction To Phase Equilibriumaa100% (1)
- 1st Law of Thermodynamics-3 PDFDocument9 pages1st Law of Thermodynamics-3 PDFSahmi Abdulqahar NizoriNo ratings yet
- Formelsammlung - ZustandsänderungenDocument2 pagesFormelsammlung - ZustandsänderungenTrần Nguyên KhôiNo ratings yet
- Termodinamica ch03Document35 pagesTermodinamica ch03Rebeca AlmeidaNo ratings yet
- ICE - Lecture From MapuaDocument48 pagesICE - Lecture From MapuaMarcial Jr. MilitanteNo ratings yet
- U1-Lab - Munguia Pinacho Isai SalatielDocument10 pagesU1-Lab - Munguia Pinacho Isai SalatielMunguia SalatielNo ratings yet
- Sasi Institute of Technology EnineeringDocument6 pagesSasi Institute of Technology EnineeringHamu NalaNo ratings yet
- ME 331 Thermodynamics II Lecture 3cDocument31 pagesME 331 Thermodynamics II Lecture 3cJosell CaipangNo ratings yet