Professional Documents
Culture Documents
Chapter 9, Biols300
Uploaded by
mariamOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 9, Biols300
Uploaded by
mariamCopyright:
Available Formats
CHAPTER 9
Mitochondrial Structure and Function
© 2013 John Wiley & Sons, Inc. All rights reserved.
1
Keys
• Clarify the overall mitochondrial structure and function.
• Describe the inner/outer mitochondrial membranes and matrix.
• Review the processes of glycolysis and anaerobic fermentation.
• Emphasize the electron transport system in forming a proton gradient.
• Describe the chemiosmotic mechanism and cellular energy transduction.
• Clarify mechanisms for transporting proton to the intermembrane space.
• Describe the mechanisms ATP synthase may convert ADP and Pi to ATP.
• Emphasize the roles of the proton-motive force other than ATP synthesis.
• Discuss the control of respiration.
• Discuss the role of mitochondria in heat production.
• Describe the mechanisms to import proteins into the mitochondria.
• Elucidate the structure and function of peroxisomes and glyoxysomes.
© 2013 John Wiley & Sons, Inc. All rights reserved.
2
Introduction
• The early Earth was populated by anaerobes, which captured
and utilized energy by oxygen-independent metabolism.
• Oxygen accumulated in the primitive atmosphere after
cyanobacteria appeared.
• Aerobes evolved to use oxygen to extract more energy from
organic molecules.
• In eukaryotes, aerobic respiration takes place in the
mitochondrion.
© 2013 John Wiley & Sons, Inc. All rights reserved.
3
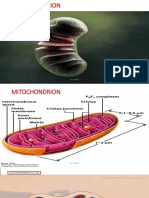
(9.1) Mitochondrial Structure and Function
Elongated mitochondria Transmission electron Mitochondria in the
of fibroblast micrograph sperm mid-piece
• Mitochondria: characteristic morphologies despite variable appearance.
– Typical mitochondria are bean-shaped organelles but may be round or
threadlike.
– Size and number of mitochondria reflect the energy requirements of the cell.
© 2013 John Wiley & Sons, Inc. All rights reserved.
4
Mitochondrial Structure and Function
Dynamic nature of
mitochondria
revealed in mouse
fibroblasts with a
fluorescent tagged
mitochondrial
protein.
• Mitochondria can fuse with one another, or split in two.
– The balance between fusion and fission is likely a major determinant
of mitochondrial number, length, and degree of interconnection.
© 2013 John Wiley & Sons, Inc. All rights reserved.
5
Mitochondrial Structure and Function
3D model of contacts Model for mitochondrial fission: ER
between ER and tubules and Drp1 mediate mitochondrial
mitochondria constriction.
• Mitochondria can fuse with one another, or split in two.
– The balance between fusion and fission is likely a major determinant
of mitochondrial number, length, and degree of interconnection.
© 2013 John Wiley & Sons, Inc. All rights reserved.
6
Mitochondrial Structure and Function
The structure of a mitochondrion
• Inner and outer mitochondrial Scanning
membranes enclose two electron
spaces: the matrix and micrograph of
a macerated
intermembrane space.
mitochondrion
– The outer mitochondrial
membrane serves as its outer
boundary.
– The inner mitochondrial 3D
membrane is subdivided into reconstruction of
two interconnected domains: a mitochondrion
based on a
• Inner boundary
micrographs
membrane
taken with a
• Cristae: where the high-voltage
machinery for ATP is electron
located microscope
© 2013 John Wiley & Sons, Inc. All rights reserved.
7
Mitochondrial Structure and Function
The structure of a mitochondrion
• Outer mitochondrial
membrane: outer boundary.
• Inner mitochondrial
membrane has two
interconnected domains:
• Inner boundary membrane
• Cristae: where the
machinery for ATP is located
• The mitochondrial matrix
– Contains a circular DNA
molecule, ribosomes, and
enzymes.
– RNA and proteins can be Schematic diagrams showing the 3D
synthesized in the matrix. internal structure and a thin section of a
mitochondrion from bovine heart tissue
© 2013 John Wiley & Sons, Inc. All rights reserved.
8
Mitochondrial Structure and Function
• Mitochondrial Membranes
– The outer membrane is about 50%
lipid; the inner membrane is more
than 75% protein.
– The inner membrane contains
cardiolipin but not cholesterol,
both are true of bacterial
membranes.
– The outer membrane contains a
large pore-forming protein called
porin.
– The inner membrane is
impermeable to even small
molecules; the outer membrane is Porin motif: a β-sheet barrel that
permeable to even some proteins. forms an opening for passage of
moderate-sized molecules
© 2013 John Wiley & Sons, Inc. All rights reserved.
9
Mitochondrial Structure and Function
• Mitochondrial Membranes
– The outer membrane is about 50%
lipid; the inner membrane is more
than 75% protein.
– The inner membrane contains
cardiolipin but not cholesterol,
both are true of bacterial
membranes.
– The outer membrane contains a
large pore-forming protein called
porin.
– The inner membrane is
impermeable to even small
molecules; the outer membrane is Porin motif: a β-sheet barrel that
permeable to even some proteins. forms an opening for passage of
moderate-sized molecules
© 2013 John Wiley & Sons, Inc. All rights reserved.
10
Mitochondrial Structure and Function
Carbohydrate metabolism in eukaryotic cells
Coupling cytosolic glycolysis and pyruvate production
to the mitochondrial TCA cycle and ATP formation
© 2013 John Wiley & Sons, Inc. All rights reserved.
11
(9.2) Oxidative Metabolism in the
Mitochondrion
• The first steps in
oxidative metabolism are
carried out in glycolysis.
– Glycolysis produces
pyruvate, NADH, and
two molecules of ATP.
– Aerobic organisms use
O2 to extract more than
30 additional ATPs from
pyruvate and NADH.
– Pyruvate is transported
across the inner
membrane and
decarboxylated to form
Coupling cytosolic glycolysis and pyruvate production
acetyl CoA, which enters
to the mitochondrial TCA cycle and ATP formation
the next stage.
© 2013 John Wiley & Sons, Inc. All rights reserved.
12
Oxidative Metabolism in the Mitochondrion
An overview of glycolysis
Overview of glycolysis
showing some of the
key steps.
© 2013 John Wiley & Sons, Inc. All rights reserved.
13
Oxidative Metabolism in the Mitochondrion
The TCA cycle
• The tricarboxylic acid (TCA)
cycle
– It is a stepwise cycle where
substrate is oxidized and its
energy conserved.
– The two-carbon acetyl group
from acetyl CoA is condensed
with the four-carbon
oxaloacetate to form a six-
carbon citrate.
– During the cycle, two carbons
are oxidized to CO2, regenerating
the four-carbon oxaloacetate
needed to continue the cycle.
© 2013 John Wiley & Sons, Inc. All rights reserved.
14
Oxidative Metabolism in the Mitochondrion
The TCA cycle
• The TCA cycle
– Four reactions in the
cycle transfer a pair
of electrons to NAD+
to form NADH, or to
FAD+ to form FADH2.
– Reaction
intermediates in the
TCA cycle are
common compounds
generated in other
catabolic reactions
making the TCA cycle
the central metabolic
pathway of the cell. Catabolic pathways generate compounds
that are fed into the TCA cycle
© 2013 John Wiley & Sons, Inc. All rights reserved.
15
Oxidative Metabolism in the Mitochondrion
The glycerol phosphate shuttle
• The Importance of
Reduced Coenzymes in
the Formation of ATP
– The reduced coenzymes
FADH2 and NADH are the
primary products of the
TCA cycle.
– NADH formed during
glycolysis enters the
mitochondria via malate-
aspartate or glycerol
phosphate shuttles.
Electron transfer from NADH to DHAP to form
glycerol 3-phosphate, then to FAD to form FADH2
© 2013 John Wiley & Sons, Inc. All rights reserved.
16
Oxidative Metabolism in the Mitochondrion
Summary of oxidative phosphorylation
• Importance of Reduced Coenzymes
– As electrons move through the
electron-transport chain, H+ are
pumped out across the inner
membrane.
– ATP is formed by the controlled
movement of H+ back across the
membrane through the ATP-
synthesizing enzyme.
– Coupling of H+ translocation to ATP
synthesis is called chemiosmosis.
– Three molecules of ATP are formed
from each pair of electrons donated
by NADH; two molecules of ATP are Two step process of oxidative
formed from each pair of electrons phosphorylation: Formation and
donated by FADH2. harnessing of the proton gradient
© 2013 John Wiley & Sons, Inc. All rights reserved.
17
The Role of Mitochondria in the
Formation of ATP
• Electron Transport
– Electrons move through
the inner membrane via a
series of carriers of
decreasing redox
potential.
– Electrons associated with
either NADH or FADH2 are
transferred through
specific electron carriers
that make up the
electron transport chain.
Redox potential of some reaction couples
© 2013 John Wiley & Sons, Inc. All rights reserved.
18
The Role of Mitochondria in the
Formation of ATP
• Types of Electron Carriers
– Flavoproteins are
polypeptides bound to either
FAD or FMN.
– Cytochromes contain heme
groups bearing Fe or Cu metal
ions.
– Three cooper atoms are
located within a single protein
complex and alternate
between Cu2+/Cu3+
– Ubiquinone (coenzyme Q) is a
lipid-soluble molecule made
of five-carbon isoprenoid
units.
Structures of three electron carriers
© 2013 John Wiley & Sons, Inc. All rights reserved.
19
The Role of Mitochondria in the
Formation of ATP
Iron-sulfur centers Arrangement of several carriers Inhibitors to determine
in the electron-transport chain the ETC carrier sequence
• Types of Electron Carriers
– Iron-sulfur proteins contain Fe in association with inorganic sulfur.
– These carriers are arranged in order of increasingly positive redox potential.
– Sequence of carriers determined by use if inhibitors.
© 2013 John Wiley & Sons, Inc. All rights reserved.
20
The Role of Mitochondria in the
Formation of ATP
• Electron-Transport Complexes
– Complex I (NADH dehydrogenase) catalyzes transfer of electrons from
NADH to ubiquinone and transports four H+ per pair.
– Complex II (succinate dehydrogenase) catalyzes transfer of electrons from
succinate to FAD to ubiquinone without transport of H+.
– Complex III (cytochrome bc1) catalyzes the transfer of electrons from
ubiquinone to cytochrome c and transports four H+ per pair.
– Complex IV (cytochrome c oxidase) catalyzes transfer of electrons to O2
and transports H+ across the inner membrane.
– Cytochrome oxidase is a large complex that adds four electrons to O2 to
form two molecules of H2O.
– The metabolic poisons CO, N3–, and CN– bind catalytic sites in Complex IV.
© 2013 John Wiley & Sons, Inc. All rights reserved.
21
The Role of Mitochondria in the
Formation of ATP
The electron-transport chain of the inner mitochondrial membrane
© 2013 John Wiley & Sons, Inc. All rights reserved.
22
The Role of Mitochondria in the
Formation of ATP
cytochrome oxidase:
A proton pump in
synthetic liposomes
Model of electron
flow through the
four redox centers
– Cytochrome oxidase is a large complex that adds four electrons to O2 to
form two molecules of H2O.
– Electrons are transferred one at a time.
– Energy released by O2 reduction is thought to drive conformational changes.
– These changes would promote H+ ions movement through the protein.
© 2013 John Wiley & Sons, Inc. All rights reserved.
23
(9.4) Translocation of Protons and the
Establishment of a Proton-Motive Force
• Two components of the proton gradient:
– Concentration gradient between matrix
and intermembrane space creates a pH
gradient (ΔpH).
– Separation of charge across the membrane
creates an electric potential (Ψ).
– Energy present in both components of the
gradients is proton-motive force (Δp).
Visualizing the proton-motive
force in active mitochondria
with the fluorescent, cationic
dye rhodamine
© 2013 John Wiley & Sons, Inc. All rights reserved.
24
The Machinery for ATP Formation
The structure of the ATP synthase
Schematic
diagram and 3D
structure of the
bacterial ATP
synthase. The
enzyme consists
of two major
portions, called
F1 and F0
• The structure of the ATP synthase:
– The F1 particle is the catalytic subunit, and contains three catalytic sites for
ATP synthesis.
– The F0 particle attaches to the F1 and is embedded in the inner membrane.
– The F0 base contains a channel through which protons are conducted from the
intermembrane space to the matrix–demonstrated in experiments with sub-
mitochondrial particles.
© 2013 John Wiley & Sons, Inc. All rights reserved.
25
The Machinery for ATP Formation
The structure of the ATP synthase
Schematic diagram of the
bacterial ATP synthase.
Atomic force
microscopy of a
“field” of c rings
from chloroplast
ATP synthases
with 14 subunits
• The structure of the ATP synthase:
– The number of subunits in the c ring is 10–14 because structural studies have
revealed that this number can vary depending on the source of the enzyme.
– Yeast mitochondrial and E. coli ATP synthase have 10 c subunits.
– The chloroplast ATP synthase has 14 c subunits.
© 2013 John Wiley & Sons, Inc. All rights reserved.
26
The Machinery for ATP Formation
Binding Change Mechanism
A section through the F1
head shows the spatial
organization of three of
its subunits
Top view of the F1 head
with the six α and β
subunits around the
asymmetric γ subunit
• The Basis of ATP Formation According to the Binding Change Mechanism
– The binding change mechanism states the following:
• Movement of protons through ATP synthase alters the binding affinity of
the active site.
• Each active site goes through distinct conformations that have different
affinities for substrates and product.
• There is a structural basis of catalytic site conformation.
© 2013 John Wiley & Sons, Inc. All rights reserved.
27
The Machinery for ATP Formation
Binding Change Mechanism
The binding
change
mechanism
for ATP
synthesis.
• Binding change mechanism
– Binding sites on the catalytic subunit can be tight, loose, or open.
– ATP is synthesized through rotational catalysis where the stalk of ATP synthase
rotates relative to the head.
– There is structural and experimental evidence to support this mechanism.
© 2013 John Wiley & Sons, Inc. All rights reserved.
28
The Machinery for ATP Formation
• Using the Proton Gradient to
Drive the Catalytic Machinery:
The Role of the F0 Portion of ATP
Synthase
– The c subunits of the F0 base
form a ring.
– The c ring is bound to γ subunit
of the stalk.
– Protons moving through
membrane rotate the ring.
– Rotation of the ring provides
twisting force that drives ATP
synthesis.
A model of the proton diffusion coupled
to rotation of c ring in the F0 complex
© 2013 John Wiley & Sons, Inc. All rights reserved.
29
The Machinery for ATP Formation
• Other Roles for the Proton-
Motive Force in Addition to
ATP Synthesis
– The H+ gradient drives
transport of ADP into and
ATP out of the
mitochondrion.
– ADP is the most important
factor controlling the
respiration rate.
– Many factors influence
the rate of respiration, but
the pathways are poorly
understood.
Summary of the major activities during
aerobic respiration in a mitochondrion.
© 2013 John Wiley & Sons, Inc. All rights reserved.
30
Copyright 2013 John Wiley & Sons, Inc.
All rights reserved. Reproduction or translation of this work
beyond that permitted in section 117 of the 1976 United
States Copyright Act without express permission of the
copyright owner is unlawful. Request for further information
should be addressed to the Permissions Department, John
Wiley & Sons, Inc. The purchaser may make back-up copies
for his/her own use only and not for distribution or resale.
The Publisher assumes no responsibility for errors,
omissions, or damages caused by the use of these programs
or from the use of the information herein.
© 2013 John Wiley & Sons, Inc. All rights reserved.
31
You might also like
- Gene TherapyDocument31 pagesGene TherapySriram RNo ratings yet
- Target Identification and ValidationDocument21 pagesTarget Identification and ValidationManuel Christopher MontesclarosNo ratings yet
- Biols102 All ChaptersDocument617 pagesBiols102 All ChaptersmariamNo ratings yet
- DNA Replication WorksheetDocument2 pagesDNA Replication Worksheetfabyunaaa100% (1)
- Unit 2: Simplified Lecture NotesDocument88 pagesUnit 2: Simplified Lecture NotesMohmmed tajNo ratings yet
- Pharma and Life Sciences List Telangana & AP - With Contacts Upto CEODocument20 pagesPharma and Life Sciences List Telangana & AP - With Contacts Upto CEOVicky SinhaNo ratings yet
- Chapter 9 PDFDocument44 pagesChapter 9 PDFFiona SimeonNo ratings yet
- Mitochondrion PresentationDocument9 pagesMitochondrion Presentationv7b4h2dxxnNo ratings yet
- Biology-2022-2024 SyllabusDocument34 pagesBiology-2022-2024 SyllabusAqsa KhalilNo ratings yet
- BIO Reviewer - All Abt CellsDocument5 pagesBIO Reviewer - All Abt CellsLeilah Angelique KunkelNo ratings yet
- L 4 Cell Organization IIDocument35 pagesL 4 Cell Organization IIsNo ratings yet
- BIO105P Module 1 Part 2Document39 pagesBIO105P Module 1 Part 2Sinajh May Solomon CabalNo ratings yet
- 2022 2024 SyllabusDocument33 pages2022 2024 Syllabus15jirantanin TapNo ratings yet
- Biology 12 - Cell Structure & Function: Chapter Notes: The Cell Theory Can Be Summarized AsDocument7 pagesBiology 12 - Cell Structure & Function: Chapter Notes: The Cell Theory Can Be Summarized AsRahul PNo ratings yet
- Silibus Topic 1Document2 pagesSilibus Topic 1Muhd Rahmat Moktar LotfyNo ratings yet
- CellDocument5 pagesCellhkrybmzxfbxbwnpfhnNo ratings yet
- Cell Lecture 3Document18 pagesCell Lecture 3haamajansiNo ratings yet
- Chem 142 - N Chapter 1 CellsDocument10 pagesChem 142 - N Chapter 1 CellsDaniel Joshua MiguelNo ratings yet
- MITOCHONDRIADocument15 pagesMITOCHONDRIAKhurram HussainNo ratings yet
- General Characteristics: of Mitochondria 1 Methods of StudyDocument7 pagesGeneral Characteristics: of Mitochondria 1 Methods of StudypaulaNo ratings yet
- The Cell StudentDocument90 pagesThe Cell Studentmaricar basilanNo ratings yet
- Lecture 3Document22 pagesLecture 3raja11160No ratings yet
- Grade 12 (P) Scheme of Work 2023Document14 pagesGrade 12 (P) Scheme of Work 2023ramloghun veerNo ratings yet
- Grade 12 (P) Scheme of Work 2023 NewDocument15 pagesGrade 12 (P) Scheme of Work 2023 Newramloghun veerNo ratings yet
- Cell Molecular Composition of CellDocument72 pagesCell Molecular Composition of Celljomel rondinaNo ratings yet
- Genetics Notes Week 1-3Document15 pagesGenetics Notes Week 1-3SOFHIA CLAIRE SUMBAQUILNo ratings yet
- A CelulaDocument22 pagesA CelulaNerea TojeiroNo ratings yet
- Cell OrganellesDocument63 pagesCell Organellessyafi zulNo ratings yet
- Mpar Chapter 2Document6 pagesMpar Chapter 2Sophia LayugNo ratings yet
- Kuliah 2 - CellDocument57 pagesKuliah 2 - Cellkamalab04No ratings yet
- Anatomy & Physiology: Seeley'sDocument71 pagesAnatomy & Physiology: Seeley'sNatron PickettNo ratings yet
- 3 4. Struktur Dan Fungsi Pada Sel ProkariotDocument29 pages3 4. Struktur Dan Fungsi Pada Sel Prokariotenderx675No ratings yet
- FET CP 1 Cell Structure & FunctionsDocument220 pagesFET CP 1 Cell Structure & FunctionsFarhat GirangNo ratings yet
- BIO 35 Chapter 1B - Cell Anatomy and PhysiologyDocument11 pagesBIO 35 Chapter 1B - Cell Anatomy and PhysiologyJake EverettNo ratings yet
- Group 2. Presentation - Cell 1Document57 pagesGroup 2. Presentation - Cell 1Thông LêNo ratings yet
- Grade 12:13 (S) Scheme of Work 2023Document15 pagesGrade 12:13 (S) Scheme of Work 2023ramloghun veerNo ratings yet
- MitochondriaDocument14 pagesMitochondriaPhoebe KimNo ratings yet
- 5 A Tour of The CellDocument106 pages5 A Tour of The CellQuino AmarelaNo ratings yet
- MitochindriaDocument8 pagesMitochindriaAhmad AliNo ratings yet
- 2 Ultra Structure of CellsDocument49 pages2 Ultra Structure of CellsYousef WardatNo ratings yet
- Chapter 7 Cell Structure and FunctionDocument62 pagesChapter 7 Cell Structure and Function310730기김민준No ratings yet
- Chapter 1Document67 pagesChapter 1Serra ÖzışıkNo ratings yet
- 2 A - The-CellDocument18 pages2 A - The-CellSharmain Pranne Dulnuan NgipolNo ratings yet
- Chapter 7 Lecture: Cell BiologyDocument86 pagesChapter 7 Lecture: Cell BiologyQueenie Anne Barroga AspirasNo ratings yet
- BioAct Chapter 2 - QuestionDocument44 pagesBioAct Chapter 2 - QuestionNOOR JANNAH FIRDOUSE BINTI ISMAIL KM-PensyarahNo ratings yet
- Botany LAB Plant and Animal CellsDocument7 pagesBotany LAB Plant and Animal CellsJasmine SandroNo ratings yet
- 1 Introduction To Cell Biology: 1.1 MotivationDocument8 pages1 Introduction To Cell Biology: 1.1 MotivationjohnnyNo ratings yet
- Chromasomal Basis of HeredityDocument9 pagesChromasomal Basis of Heredityplmbsbio1No ratings yet
- History and Features of Eukaryotic Cell (Part1)Document31 pagesHistory and Features of Eukaryotic Cell (Part1)Ally AbadeoNo ratings yet
- Chem123 - PrelimsDocument20 pagesChem123 - Prelimslily of the valleyNo ratings yet
- The Structure and Function of The MitochondrionDocument1 pageThe Structure and Function of The MitochondrionSebastiancody PasuquinNo ratings yet
- Study MaterialDocument364 pagesStudy MaterialNaeem AbbasNo ratings yet
- 06-The Eukaryotic CellDocument76 pages06-The Eukaryotic Celldelia selmiNo ratings yet
- Ultra-structure-of-Mitochondria-Semester I F. Y. B.Sc.Document16 pagesUltra-structure-of-Mitochondria-Semester I F. Y. B.Sc.mbhattacharya094956No ratings yet
- (H2 Bio) Chapter 1 Cell BiologyDocument29 pages(H2 Bio) Chapter 1 Cell BiologywengiemotshegweNo ratings yet
- IB Better - Biology SyllabusDocument19 pagesIB Better - Biology SyllabusVaishnav VinodNo ratings yet
- Mitochondria - The Powerhouses of The CellDocument1 pageMitochondria - The Powerhouses of The CellVinícius SouzaNo ratings yet
- CellsDocument6 pagesCellsaryam aliNo ratings yet
- Optional - Cellular Level ofDocument24 pagesOptional - Cellular Level ofdafabc50No ratings yet
- L 2 Cell Structure and FunctionDocument25 pagesL 2 Cell Structure and Functionkaukab azimNo ratings yet
- MitochondriaDocument13 pagesMitochondriamariemmohamed200713No ratings yet
- Chapter 3. Cell Structure and Taxonomy ReviewerDocument7 pagesChapter 3. Cell Structure and Taxonomy ReviewerAlexandra MalagaNo ratings yet
- Semi Autonomus Nature of Mitochondria 1st YearDocument9 pagesSemi Autonomus Nature of Mitochondria 1st YearTalha ArshadNo ratings yet
- Cell & Binomial NomenclatureDocument6 pagesCell & Binomial NomenclatureVenice De los ReyesNo ratings yet
- From Tubulin to Thought: The Nexus of Cytoskeleton Microtubules and Brain Complexity.From EverandFrom Tubulin to Thought: The Nexus of Cytoskeleton Microtubules and Brain Complexity.No ratings yet
- CH 27Document92 pagesCH 27mariamNo ratings yet
- CH 24Document71 pagesCH 24mariamNo ratings yet
- CH 14Document126 pagesCH 14mariamNo ratings yet
- CH 26Document46 pagesCH 26mariamNo ratings yet
- CH 15Document54 pagesCH 15mariamNo ratings yet
- CH 25Document61 pagesCH 25mariamNo ratings yet
- CH 13Document120 pagesCH 13mariamNo ratings yet
- BIOLS 300 Lab 3 - Thin Layer Chromatography PowerpointDocument38 pagesBIOLS 300 Lab 3 - Thin Layer Chromatography PowerpointmariamNo ratings yet
- Chapter 10, Biols300Document26 pagesChapter 10, Biols300mariamNo ratings yet
- Chapter 8 Cellular Membranes Part 2, Biols300Document46 pagesChapter 8 Cellular Membranes Part 2, Biols300mariamNo ratings yet
- Chapter 2 The Structure and Functions of Biological Molecules, Biols300Document99 pagesChapter 2 The Structure and Functions of Biological Molecules, Biols300mariamNo ratings yet
- Chapter 1 Introduction To Cell Biology, Biols300Document38 pagesChapter 1 Introduction To Cell Biology, Biols300mariamNo ratings yet
- HW8 - Metabolism 1Document2 pagesHW8 - Metabolism 1Christopher GalivoNo ratings yet
- Genetics: The Genetic MaterialDocument25 pagesGenetics: The Genetic Materialعمار مصعب عادلNo ratings yet
- Characterization of A Epilactose-Productiong Cellobiose 2-Epimerase From Thermoanaerobacterium SacharolyticumDocument6 pagesCharacterization of A Epilactose-Productiong Cellobiose 2-Epimerase From Thermoanaerobacterium SacharolyticumKukymovNo ratings yet
- Cell Membrane Worksheet 1 PDFDocument4 pagesCell Membrane Worksheet 1 PDFVidanes, Earl JosephNo ratings yet
- BIOLOGY Topic 1 Past Paper QuestionsDocument9 pagesBIOLOGY Topic 1 Past Paper QuestionsLoraine NNo ratings yet
- Option B BiochemistryDocument65 pagesOption B BiochemistryIrene RomeroNo ratings yet
- IB Biology Revision Spreadsheet - Cell BioDocument8 pagesIB Biology Revision Spreadsheet - Cell Biopari meharunkarNo ratings yet
- Bdwbu 14: Kinetic EnergyDocument22 pagesBdwbu 14: Kinetic Energyzahid KhanNo ratings yet
- Bio1 11 - 12 Q1 0201 PF FDDocument39 pagesBio1 11 - 12 Q1 0201 PF FDJomana MacalnasNo ratings yet
- Module 3 Section 3Document8 pagesModule 3 Section 3Grace CumamaoNo ratings yet
- Fpubh 02 00103Document9 pagesFpubh 02 00103Puja AgestiNo ratings yet
- HPV Qual PCRDocument53 pagesHPV Qual PCRyousrazeidan1979No ratings yet
- Application of Recombinant DNATechnologies On Sub-Cloning of Transcriptional Co-FactorDocument6 pagesApplication of Recombinant DNATechnologies On Sub-Cloning of Transcriptional Co-FactorInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- DNA The Molecule of LifeDocument37 pagesDNA The Molecule of LifeYanuar PudjihardjoNo ratings yet
- 3 Cell Division QDocument6 pages3 Cell Division QsyukriNo ratings yet
- La Celula EstresadaDocument9 pagesLa Celula EstresadaPedro CandelarioNo ratings yet
- Ahmed-Kovinich2021 Article RegulationOfPhytoalexinBiosyntDocument23 pagesAhmed-Kovinich2021 Article RegulationOfPhytoalexinBiosyntluthfiana mimiftaNo ratings yet
- JCR and Journal Quart I Les 2022Document1,275 pagesJCR and Journal Quart I Les 2022Natalie CheongNo ratings yet
- Pre PSPM QDocument4 pagesPre PSPM QCarin TanNo ratings yet
- 06 01 Electrophoresis Lab ReportDocument3 pages06 01 Electrophoresis Lab ReportYunseo LazNo ratings yet
- 1.2 Genes - Answers & QuestionsDocument6 pages1.2 Genes - Answers & QuestionsKristina LallNo ratings yet
- Taq DNA Polymerase 1.1x Master Mix: ProtocolDocument2 pagesTaq DNA Polymerase 1.1x Master Mix: ProtocolsharonNo ratings yet
- Blue Biotechnology: Home (/default - Aspx) Books Search (/advancedsearch - Aspx) Support About Us Get PublishedDocument18 pagesBlue Biotechnology: Home (/default - Aspx) Books Search (/advancedsearch - Aspx) Support About Us Get Publishedcarlos esparzaNo ratings yet
- TissUse Product CatalogDocument36 pagesTissUse Product CatalogHui Ling MaNo ratings yet
- Non-Clinical AssessmentDocument37 pagesNon-Clinical AssessmentacbgdvNo ratings yet
- Animal Models of Alzheimer's DiseaseDocument11 pagesAnimal Models of Alzheimer's DiseaseTapan K. NayakNo ratings yet