Professional Documents
Culture Documents
SolvedProblem PFR VariableVolumeReaction
Uploaded by
Harish PrasathOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
SolvedProblem PFR VariableVolumeReaction
Uploaded by
Harish PrasathCopyright:
Available Formats
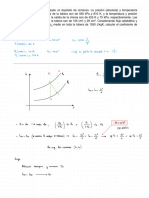
H◦d-ks
sfnrlriomehy A → R for first order gasphase
Assuming
a
a
pure A
is
for 99% conversion of
reaction the size of a PFR
stoichiometry of tse
,
liters In fact, the
calculated G be 32 .
3K For this corrected stoichiometry, find the
reaction is A → .
volume of
reactor
required
.
PFR design → natron :
÷ -11¥
- YA = Kca
a- =
ⁿʰ✓=%%¥a) = ↳
!Ie±a✗a
CAG =
kG¥¥¥
-
ra = Kca =
✗A
÷=!¥÷÷:-:I ,
r¥F=%?¥: →
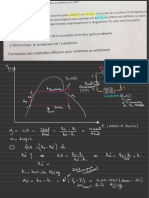
For A → R Glen :
Ea = ◦
✗a- = 0.99
✗A
VK.ph?---f9II-a---ln( 0
c- ✗ a)
✓=
32lb & ✗A = 0.99
Given :
32kq§0_ In (1-0.99)
'
• . = -
k÷ =
4¥52 = 0.144
For A → 3. R
Ea = = 2
substituting the above in F-qn.IO
,
vi-o.ae =
. )
✗A
=*¥:
0
✗A ✗A
.fi#--J?II-ad*
✗A ✗A
↓% +214¥ *
-
1) ᵈ✗a
✗A
✗A
=3f• - &
/ d✗*
°
0
° '
=
@ lace -
a)
✗ - 2✗ a)
0
= -
3 en@ .
-
1) - 2×0^99
= 13.816 -
1.98 = 11.84
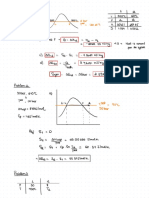
r =
18.94-a ᵗr ,
=
You might also like
- TermoDocument5 pagesTermoFederiko NainggolanNo ratings yet
- 25.10.2023 C01Document9 pages25.10.2023 C01robert lawrenceNo ratings yet
- 20Document12 pages20Rajat Verma X D 39No ratings yet
- Clase 20220530 FQDocument11 pagesClase 20220530 FQJeshNo ratings yet
- Levenspiel 5 19 MFR KineticsDocument1 pageLevenspiel 5 19 MFR KineticsHarish PrasathNo ratings yet
- Solutions & Colligative PropertiesDocument14 pagesSolutions & Colligative PropertiesPoonam PrasadNo ratings yet
- Metodos IntegralesDocument31 pagesMetodos IntegralesJosé Arturo FerreraNo ratings yet
- Capacitors Live Class-5 Teacher NotesDocument19 pagesCapacitors Live Class-5 Teacher Notessaksham jainNo ratings yet
- Non-Isothermal Reactor DesignDocument5 pagesNon-Isothermal Reactor Designnorpius7754No ratings yet
- Electrostatics Revision NotesDocument45 pagesElectrostatics Revision NotescjpwuiNo ratings yet
- Lecture 3A - Analysis of Batch Reactors (Simple Reactions) Aa - P PDFDocument6 pagesLecture 3A - Analysis of Batch Reactors (Simple Reactions) Aa - P PDFPAMELA TEJADANo ratings yet
- CEN444 HW4 Sila Sarochananjeen 6005490Document3 pagesCEN444 HW4 Sila Sarochananjeen 6005490MAY THWE HTUNNo ratings yet
- CHBE 355 Assignment 3 SolnDocument7 pagesCHBE 355 Assignment 3 SolnAwesome GeneralNo ratings yet
- 11.10.2023 C01Document8 pages11.10.2023 C01robert lawrenceNo ratings yet
- Homework 2Document6 pagesHomework 2Prakhar AgrawalNo ratings yet
- Solid State Problem Solving Class Teacher NotesDocument37 pagesSolid State Problem Solving Class Teacher Notesbalrama sharmaNo ratings yet
- ProblemasDocument3 pagesProblemasJuan Carlos P. R.No ratings yet
- Remaining ProblemsDocument4 pagesRemaining ProblemsKyokyo TokimiNo ratings yet
- Emailing Chemical Kinetics (Class 12)Document12 pagesEmailing Chemical Kinetics (Class 12)Bakul ShrivastavaNo ratings yet
- Emailing Chemical Kinetics (Class 12)Document12 pagesEmailing Chemical Kinetics (Class 12)roceniNo ratings yet
- Final CheatsheetDocument4 pagesFinal CheatsheetmehulbasuNo ratings yet
- " Gradient: 2ha enDocument2 pages" Gradient: 2ha enAkanksh SubramanyaNo ratings yet
- Trigonometry NotesDocument1 pageTrigonometry NotesRainbow100% (1)
- Week 4 - Isothermal Reactor Design (Part I)Document51 pagesWeek 4 - Isothermal Reactor Design (Part I)NadineNo ratings yet
- Exo Thermos 2emsessDocument12 pagesExo Thermos 2emsessإبراهيم الزايدي الكيحلNo ratings yet
- Waves Revision Class Teacher NotesDocument21 pagesWaves Revision Class Teacher NotesRAKSHIT GOYALNo ratings yet
- Problem 3.44 PDFDocument2 pagesProblem 3.44 PDFKauê BrittoNo ratings yet
- Assignment 9 SolutionsDocument8 pagesAssignment 9 SolutionsClerry SamuelNo ratings yet
- 29 05 ElivDocument13 pages29 05 ElivTapan BadheiNo ratings yet
- Tutorial AicDocument2 pagesTutorial AicAdarsh RNo ratings yet
- Chapter 25 TEST BANKDocument18 pagesChapter 25 TEST BANKAli AlhammadiNo ratings yet
- Mathematics NotesDocument15 pagesMathematics NotesZIXU CHEAHNo ratings yet
- CombinăriiDocument13 pagesCombinăriiskfbsdfhbNo ratings yet
- p.3 API Hydraulics EquationsDocument2 pagesp.3 API Hydraulics Equationsnasir.hdip8468No ratings yet
- Angle Measures in Degrees MUST Have A Degree Symbol. Angle Measure in Radians, Have No SymbolDocument3 pagesAngle Measures in Degrees MUST Have A Degree Symbol. Angle Measure in Radians, Have No Symboljoudialrama004No ratings yet
- 1 2 3 4 5 MergedDocument5 pages1 2 3 4 5 Mergedviki14No ratings yet
- Fined: OfmdesofdedrDocument9 pagesFined: OfmdesofdedrAkash.SNo ratings yet
- Equation Sheet HeatDocument5 pagesEquation Sheet HeatNoor GoldNo ratings yet
- F-Riviera: AlishaDocument5 pagesF-Riviera: AlishaFalisha RivienaNo ratings yet
- Exercise (Sedimentation) PDFDocument20 pagesExercise (Sedimentation) PDFDivyashini MohanNo ratings yet
- Numerical Methods and ProgrammingDocument197 pagesNumerical Methods and Programmingsatyajeetsince2001No ratings yet
- 81417-127662-Straight Line Graphs - HomeworkDocument8 pages81417-127662-Straight Line Graphs - Homework42. Nguyễn Huyền TrânNo ratings yet
- Metallurgy: For OurDocument45 pagesMetallurgy: For OurERRNo ratings yet
- Torsion Acc. RoarksDocument2 pagesTorsion Acc. RoarksAntonioNo ratings yet
- HW 12Document2 pagesHW 12haiNo ratings yet
- Be184p m1 SolDocument2 pagesBe184p m1 SolDen CelestraNo ratings yet
- Clase 20220526 FQDocument10 pagesClase 20220526 FQJeshNo ratings yet
- ข้อสอบ O-net ปี 2560-2563 เรื่อง ลำดับและอนุกรม โดย รศ.ดร.นิศากร สังวาระนทีDocument8 pagesข้อสอบ O-net ปี 2560-2563 เรื่อง ลำดับและอนุกรม โดย รศ.ดร.นิศากร สังวาระนทีKanchana PomkhamNo ratings yet
- GE ETEP TD280 NotesDocument5 pagesGE ETEP TD280 Notesabdel.bouyakhlefNo ratings yet
- Kinetics 3Document14 pagesKinetics 3Ayushman GuptaNo ratings yet
- Ejercicios de Análisis Vectorial 1) 4)Document15 pagesEjercicios de Análisis Vectorial 1) 4)Carlos DesaNo ratings yet
- Ejercicios de Análisis Vectorial 1) 4)Document9 pagesEjercicios de Análisis Vectorial 1) 4)Carlos DesaNo ratings yet
- Pet TIE 901 6Document43 pagesPet TIE 901 6Karin GergelyováNo ratings yet
- Assignment 5Document7 pagesAssignment 5Ryan ArcherNo ratings yet
- BC Notes - Series Test and TalyorDocument28 pagesBC Notes - Series Test and TalyorJiwon ShinNo ratings yet
- Kolos Z Dysku 1Document5 pagesKolos Z Dysku 1Karo JuskowiakNo ratings yet
- Reaction: 50 Absorbed)Document1 pageReaction: 50 Absorbed)zyzy6527No ratings yet
- Ap Academy 3Document3 pagesAp Academy 3José Luis Bolívar AguilarNo ratings yet
- Regmi Pramishan 3311 Midterm#1Document8 pagesRegmi Pramishan 3311 Midterm#1Pramishan RegmiNo ratings yet
- Levenspiel 5 15 CSTR VariableVolumeReactionDocument1 pageLevenspiel 5 15 CSTR VariableVolumeReactionHarish PrasathNo ratings yet
- 11plate and Frame Filter PressDocument4 pages11plate and Frame Filter PressHarish PrasathNo ratings yet
- Levenspiel 5 13 PFR VariableVolumeReactionDocument1 pageLevenspiel 5 13 PFR VariableVolumeReactionHarish PrasathNo ratings yet
- T-1'/ - L "'-D.!..S::..U..1, - :: Leyn.P &O.:H..T21EDocument24 pagesT-1'/ - L "'-D.!..S::..U..1, - :: Leyn.P &O.:H..T21EHarish PrasathNo ratings yet
- Pid Dme PlantDocument1 pagePid Dme PlantSyamsul Rizal Abd ShukorNo ratings yet
- Resistance Temperature Sensor AgingDocument10 pagesResistance Temperature Sensor Agingninoska217608No ratings yet
- Gammon India LTD - Is Not Only The Largest Civil Engineering Construction CompanyDocument4 pagesGammon India LTD - Is Not Only The Largest Civil Engineering Construction CompanyridhhhNo ratings yet
- TBM Machine UrupDocument15 pagesTBM Machine Urupdreamboy87No ratings yet
- Thermal Power - WikipediaDocument24 pagesThermal Power - WikipediaEusebia MaedzwaNo ratings yet
- Nuclear Power Plant: MR - Nitin S. Patil Sanjay Ghodawat Polytechnic, Atigre Electrical Engineering DepartmentDocument40 pagesNuclear Power Plant: MR - Nitin S. Patil Sanjay Ghodawat Polytechnic, Atigre Electrical Engineering DepartmentAnipakula DevanandNo ratings yet
- Project ReportDocument106 pagesProject ReportAnjali KumariNo ratings yet
- CIE Modern Physics Sample PagesDocument39 pagesCIE Modern Physics Sample PagesrenedavidNo ratings yet
- Iep1 20081015Document1 pageIep1 20081015jkaldeNo ratings yet
- CV Trainer SKMDocument5 pagesCV Trainer SKMsheikmoinNo ratings yet
- Reactor Control System & Reactor SafetyDocument23 pagesReactor Control System & Reactor SafetyAmol MagarNo ratings yet
- Avadhooti Aanand Masti - 3 (Vivran)Document164 pagesAvadhooti Aanand Masti - 3 (Vivran)mehaardikNo ratings yet
- Risk Management in Nuclear Power PlantsDocument4 pagesRisk Management in Nuclear Power PlantsNatália SantosNo ratings yet
- Grange IM v10-CDB (B)Document110 pagesGrange IM v10-CDB (B)Ambreen AfzalNo ratings yet
- Department of Energy - South African Energy SectorDocument12 pagesDepartment of Energy - South African Energy SectorLance Alexander WeidemanNo ratings yet
- Bhaskar - POWERDocument35 pagesBhaskar - POWERvmktptNo ratings yet
- UntitledDocument40 pagesUntitledHassan JavaidNo ratings yet
- Nuclear Power Plant Design and Seismic Safety ConsiderationsDocument34 pagesNuclear Power Plant Design and Seismic Safety ConsiderationsChuck AchbergerNo ratings yet
- Activity 1 - Energy and Power Unit ConversionDocument1 pageActivity 1 - Energy and Power Unit ConversionEkaNo ratings yet
- Ageing of Steam Generators (IAEA TECDOC-981) (1997)Document181 pagesAgeing of Steam Generators (IAEA TECDOC-981) (1997)Meliha ŠabanovićNo ratings yet
- The Oxford College of EngineeringDocument2 pagesThe Oxford College of EngineeringNarayana Swamy GNo ratings yet
- ATD RAnkin Assignment-2011Document3 pagesATD RAnkin Assignment-2011Mradul Yadav0% (2)
- Sharkawy Solution ManualDocument77 pagesSharkawy Solution ManualAl Muhallab Al KamyaniNo ratings yet
- The Windscale Reactor Accident-50 Years OnDocument6 pagesThe Windscale Reactor Accident-50 Years OnTroy LivingstonNo ratings yet
- Steam Power PlantDocument7 pagesSteam Power PlantNav Mehra50% (2)
- Dry Cooling SystemDocument53 pagesDry Cooling Systemxlxjrv50% (2)
- Nuclear Power EngineeringDocument26 pagesNuclear Power EngineeringRoed Alejandro LlagaNo ratings yet
- ps201 108 Hot CellDocument2 pagesps201 108 Hot CellKazim RazaNo ratings yet
- File 68625Document51 pagesFile 68625pumpboygrNo ratings yet
- Chapter15 PDFDocument105 pagesChapter15 PDFmohamadsadegh kamaliNo ratings yet