Professional Documents
Culture Documents
Path to success in KOTA
Uploaded by
Vedant TodiOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Path to success in KOTA
Uploaded by
Vedant TodiCopyright:
Available Formats
R
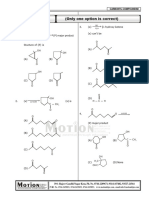
Path to success KOTA (RAJASTHAN)
Acidic strength
1. Which of the following is strongest acid?
H H
+ H + H
N N N
+ H (C) + H
(A) NH4 (B) (D)
2. What is the major product obtained from the following reaction?
COOH
HO NaOH
1 mole
NH3Cl
+
COOH .. COOH COO
–
COO—
HO —O O
(B) HO (C) . . (D) . .
—
(A)
NH2 NH3 NH3
NH3 + +
+
3. Which of the following compounds has most acidic hydrogen?
H
N
O S O
C6 H 5
(A) C6 H 5 (B) C6 H 5 (C) (D) C6 H 5
N
O S N
H
H
4. The correct order of acidic strength is/are
–
COOH COOH COOH COOH
OH OH OH OH OMe
(A) > (B) >
COOH COOH
COOH COOH
(C) > (D) >
COOH HOOC
R
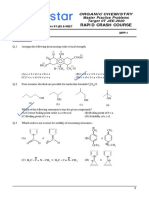
Path to success KOTA (RAJASTHAN)
5. With respect to the compounds I–V, choose the correct statement(s)
H
H H
(I) (II) (III) CH3 – H (IV) (V) H H
(A) The acidity of compound I is due to delocolisation in the conjugate base
(B) The conjugate base of compound IV is aromatic
(C) Compound II becomes more acidic, when it has a –NO2 substituent.
(D) The acidity of compounds follow the order
I > IV > V > II > III
6. The CORRECT order of acidic strength of given compound(s) is/are
OH OH OH
OCH3
(A) > >
OCH3
OCH3
H H H
N O O N O O N O
(B) > >
N
H
H H H
(C) > >
(D) HCOOH > CH3COOH > C6H5COOH
R
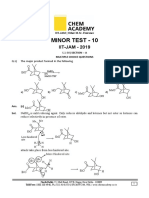
Path to success KOTA (RAJASTHAN)
Comprehension for Q.7 and Q.8
For the following compounds:
OH
OH CH 2 – OH OH
NO2
(P) (Q) (R) (S)
COOH
OH
OH
NO2 NO2
(T) H2CO3 (U) (V) CH3–OH (W)
NO2
NO2
7. Choose the possible pair(s) of binory mixture which is/are appropriate for the following scheme?
Aq.NaOH Salt of binary
mixture
Binary
mixture
Aq.NaHCO3
Compound 1 + Compound 2
(A) R and V (B) P and S (C) R and W (D) Q and U
8. Choose the correct option(s)
(A) Correct acidic strength order is U > W > T > S
(B) (S) has ‘5’ resonating structure
(C) Correct acidic strength order P > H2O > V > R
(D) Out of ‘8’ only ‘2’ an soluble in aqueous NaOH
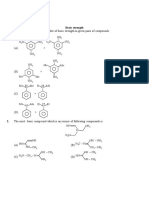
R
Path to success KOTA (RAJASTHAN)
9. Matrix Match
Column-I Column-II
Compounds pKa values
O O
(A) (P) 3.5
O
O O
(B) (Q) 10.7
O O
(C) (R) 8.9
H H
O
O
(D) N (S) 0.4
H O
10. Find the number of compound which are more acidic than H2O
OH
(1) CH3CH2–OH (2) CH3–C º CH (3) HF (4)
(5) HCl (6) CH3 – COOH (7) H2CO3 (8) HBr
(9) NH3 (10) COOH (11) CH – SH
3
You might also like
- 01 02 2023 Chemistry - Paper+With+Answer - MorningDocument6 pages01 02 2023 Chemistry - Paper+With+Answer - MorningLanaNo ratings yet
- Dap An Lev BDocument59 pagesDap An Lev BStormy StudiosNo ratings yet
- Organic Compounds Containing OxygenDocument18 pagesOrganic Compounds Containing OxygenEzhil MukilNo ratings yet
- Problem Set 1: Review Questions Chemistry 260 Organic ChemistryDocument3 pagesProblem Set 1: Review Questions Chemistry 260 Organic ChemistrydddddNo ratings yet
- 4.5 Answers To ExercisesDocument4 pages4.5 Answers To Exercisesloly62006No ratings yet
- Acids and BasesDocument30 pagesAcids and BasesSwagata SahaNo ratings yet
- General formulas and reactions in organic chemistryDocument5 pagesGeneral formulas and reactions in organic chemistryLê Minh DuyNo ratings yet
- Chapter 3 - SolutionsDocument4 pagesChapter 3 - SolutionsChronus AutomaçãoNo ratings yet
- Nanh (1 Equiv.) 2. CH - CH - I H, Lindlar Catalyst Mcpba CH Li Cubr TSCL Pyridine NacnDocument12 pagesNanh (1 Equiv.) 2. CH - CH - I H, Lindlar Catalyst Mcpba CH Li Cubr TSCL Pyridine NacnpNo ratings yet
- Sol 4Document11 pagesSol 4Minh TieuNo ratings yet
- Reaksi Aldehid Dan KetonDocument19 pagesReaksi Aldehid Dan KetonMega KurniaNo ratings yet
- Problem Set 1 PDFDocument4 pagesProblem Set 1 PDFRay BinasNo ratings yet
- Biomolecules and Polymers-02 - Solved ProblemsDocument11 pagesBiomolecules and Polymers-02 - Solved ProblemsRaju SinghNo ratings yet
- C Ch-26 BiomoleculesDocument7 pagesC Ch-26 Biomoleculesmysoftinfo.incNo ratings yet
- CarbonylDocument27 pagesCarbonylbpvx4dyqsxNo ratings yet
- 01 Roadmap SolDocument1 page01 Roadmap Solhello helloNo ratings yet
- Mechanisms 1-10: CHEM 725: Davey 1Document7 pagesMechanisms 1-10: CHEM 725: Davey 1Bradley DaveyNo ratings yet
- Amino Acids 21Document12 pagesAmino Acids 21Taha GHNo ratings yet
- Protein StructuresDocument10 pagesProtein StructuresSebastian RodriguezNo ratings yet
- LPP BiomoleculesDocument6 pagesLPP BiomoleculesAashiNo ratings yet
- BIOMOLECULESDocument6 pagesBIOMOLECULESPranav RoyNo ratings yet
- CHEM F111 General Chemistry: Electrophilic Addition ReactionDocument18 pagesCHEM F111 General Chemistry: Electrophilic Addition ReactionUtkarsh BansalNo ratings yet
- Search Results for Structures Matching Given QueriesDocument7 pagesSearch Results for Structures Matching Given QueriesEcaterina NircaNo ratings yet
- BFL F: SteplDocument2 pagesBFL F: SteplNURAISYA QALEEDA BINTI DAMAT / UPMNo ratings yet
- NMR Problems Dec 2012Document8 pagesNMR Problems Dec 2012Biswajit Gopal RoyNo ratings yet
- Figura Apendice VI Hoja 5Document1 pageFigura Apendice VI Hoja 5Andrea GalvisNo ratings yet
- Rapid Crash Course: Single CorrectDocument8 pagesRapid Crash Course: Single CorrectHudsun HornetNo ratings yet
- OH A, A Is: : CHN 1 EqvDocument4 pagesOH A, A Is: : CHN 1 EqvAtharva GanjuNo ratings yet
- Analisis Sintesis I: Sintesis Vitamin Dan Beberapa AntibiotikDocument12 pagesAnalisis Sintesis I: Sintesis Vitamin Dan Beberapa AntibiotikNovita Sari AritonangNo ratings yet
- Chemistry (Full Test) - Paper 1 - SolutionsDocument6 pagesChemistry (Full Test) - Paper 1 - SolutionsRavi Kiran KoduriNo ratings yet
- 2016 Section Module 2Document3 pages2016 Section Module 2Đạt LêNo ratings yet
- Activity 3 - The Cahn-Ingold-Prelog Rules - 0Document5 pagesActivity 3 - The Cahn-Ingold-Prelog Rules - 0Naved AnjoomNo ratings yet
- JEE (Mains) - GTM 12 - 06-01-2020Document11 pagesJEE (Mains) - GTM 12 - 06-01-2020Ravi Kiran KoduriNo ratings yet
- Advanced Biochemistry: The Krebs CycleDocument11 pagesAdvanced Biochemistry: The Krebs CycleMaritsa PerHerNo ratings yet
- Organic Chemistry: Home Assignment # 05 Topic: Biomolecule, Polymer, Chemistry in Everyday Life, Aromatic CompoundDocument12 pagesOrganic Chemistry: Home Assignment # 05 Topic: Biomolecule, Polymer, Chemistry in Everyday Life, Aromatic CompoundtuppaseeNo ratings yet
- Topic 4.8 Amino Acids Structure Acid-Base Properties Condensation Reactions ProteinsDocument8 pagesTopic 4.8 Amino Acids Structure Acid-Base Properties Condensation Reactions ProteinsSammyJayNo ratings yet
- Biomolecules 1Document7 pagesBiomolecules 1sreevaishnava01No ratings yet
- Reaksi AlkenaDocument1 pageReaksi Alkenajoel13No ratings yet
- MEKANISMEDocument2 pagesMEKANISMEEry NourikaNo ratings yet
- AP C T 7: A & B, P C: Hemistry Opic Cids Ases ARTDocument3 pagesAP C T 7: A & B, P C: Hemistry Opic Cids Ases ARTEivor LynNo ratings yet
- Organic Tutorial 1Document2 pagesOrganic Tutorial 1karthik chinnaNo ratings yet
- Sharpless Asymmetric Dihydroxylation ReactionDocument6 pagesSharpless Asymmetric Dihydroxylation ReactionHe KeNo ratings yet
- CY2102Document2 pagesCY2102Prarabdha SharmaNo ratings yet
- 19 Prac Diel Alder AnsDocument2 pages19 Prac Diel Alder AnsNguyễn ThiệnNo ratings yet
- Raj1 PDFDocument35 pagesRaj1 PDFAvdhoot Gautam100% (1)
- Chiral CompoundsDocument20 pagesChiral CompoundsShubham RampalliwarNo ratings yet
- Minor Test - 10: IIT-JAM - 2019Document37 pagesMinor Test - 10: IIT-JAM - 2019Raga NamoNo ratings yet
- Exm N X11 Chem Biomol ADocument28 pagesExm N X11 Chem Biomol Asumair hejibNo ratings yet
- Class Test Aldol and CannizaroDocument9 pagesClass Test Aldol and Cannizaroalomrobi07No ratings yet
- Org Chemistry Alcohol Carboxylic Acid Macromolecules WS AnsDocument8 pagesOrg Chemistry Alcohol Carboxylic Acid Macromolecules WS Ans2tsNo ratings yet
- Diels-Alder Reaction Practice ProblemsDocument2 pagesDiels-Alder Reaction Practice ProblemsBoas Wayne100% (1)
- Solution Manual For Organic Chemistry 7Th Edition Brown Iverson Anslyn Foote 1133952844 9781133952848 Full Chapter PDFDocument36 pagesSolution Manual For Organic Chemistry 7Th Edition Brown Iverson Anslyn Foote 1133952844 9781133952848 Full Chapter PDFrichard.parga191100% (14)
- Fisher Projection Ball-&-Stick Structure Haworth Projection Chair/Envelope Conformation Complete NameDocument3 pagesFisher Projection Ball-&-Stick Structure Haworth Projection Chair/Envelope Conformation Complete NameAmabella ShaeNo ratings yet
- Chair/Envelope and Haworth projections of monosaccharidesDocument3 pagesChair/Envelope and Haworth projections of monosaccharidesAmabella ShaeNo ratings yet
- Learning ActivityDocument3 pagesLearning ActivityAmabella ShaeNo ratings yet
- Biochem Self AssessmentDocument3 pagesBiochem Self AssessmentAmabella ShaeNo ratings yet
- C Sol Ch-23 Alcohols, Phenols and EthersDocument6 pagesC Sol Ch-23 Alcohols, Phenols and Ethersmysoftinfo.incNo ratings yet
- Biomoleculer and PolymerDocument10 pagesBiomoleculer and Polymerjiknown6No ratings yet
- Test 4 2 7 2022 PDocument9 pagesTest 4 2 7 2022 Pik62299No ratings yet
- Oc Roadmap B StudentDocument1 pageOc Roadmap B StudentVedant TodiNo ratings yet
- 07062020Document19 pages07062020Vedant TodiNo ratings yet
- 17052020Document18 pages17052020Vedant TodiNo ratings yet
- 1 Basic StrengthDocument3 pages1 Basic StrengthNidhi SharmaNo ratings yet
- 30-08-20 - INCOMING - SR - CO-SUPER CHAINA - Jee-Adv - CTA-2 - SYLLABUSDocument34 pages30-08-20 - INCOMING - SR - CO-SUPER CHAINA - Jee-Adv - CTA-2 - SYLLABUSVedant TodiNo ratings yet
- Sr.IIT_CO-SC JEE-Adv RTA-1 Practice Test QuestionsDocument31 pagesSr.IIT_CO-SC JEE-Adv RTA-1 Practice Test QuestionsVedant TodiNo ratings yet
- Telescoping RemasteredDocument11 pagesTelescoping RemasteredVedant TodiNo ratings yet
- Class XI Physics DPP Set (26) - Previous Chaps + SHMDocument24 pagesClass XI Physics DPP Set (26) - Previous Chaps + SHMVedant TodiNo ratings yet
- NSEP 2013 SolutionDocument22 pagesNSEP 2013 SolutionVedant TodiNo ratings yet
- NSEP 2018 SolutionDocument23 pagesNSEP 2018 SolutionVedant TodiNo ratings yet
- Physics Advanced Level Problem Solving (ALPS-9) - SolutionDocument12 pagesPhysics Advanced Level Problem Solving (ALPS-9) - SolutionVedant TodiNo ratings yet
- Class XI Physics DPP Set (28) - Previous Chaps + Properties of Solids & LiquidsDocument20 pagesClass XI Physics DPP Set (28) - Previous Chaps + Properties of Solids & LiquidsVedant TodiNo ratings yet
- 2022-JEE Advanced Booster Test-3 SolutionsDocument13 pages2022-JEE Advanced Booster Test-3 SolutionsVedant TodiNo ratings yet
- MOT 1 JEE 2022 Paper VMC PDFDocument10 pagesMOT 1 JEE 2022 Paper VMC PDFBiswadeep GiriNo ratings yet
- APA-1 (100Q) (Electrostatics, DC Circuits, Capacitors, Magnetism) (JEE 2020)Document22 pagesAPA-1 (100Q) (Electrostatics, DC Circuits, Capacitors, Magnetism) (JEE 2020)Vedant TodiNo ratings yet
- APA-1A (124Q+A) (Mechanics)Document18 pagesAPA-1A (124Q+A) (Mechanics)Vedant TodiNo ratings yet
- 2022-JEE Advanced Booster Test-2 PaperDocument16 pages2022-JEE Advanced Booster Test-2 PaperVedant TodiNo ratings yet
- Hawaiian Hibiscus Facts: Care, Features & Endangered StatusDocument2 pagesHawaiian Hibiscus Facts: Care, Features & Endangered StatusJustine Airra OndoyNo ratings yet
- Network Marketing BusinessesDocument1 pageNetwork Marketing BusinessessukhberNo ratings yet
- SO311222Document25 pagesSO311222Fiqri RismainiNo ratings yet
- MEP Final Corrected 2Document17 pagesMEP Final Corrected 2Prakhyati RautNo ratings yet
- Why Check Valves SlamDocument2 pagesWhy Check Valves SlamBramJanssen76No ratings yet
- 03-Security of Tenure PDFDocument2 pages03-Security of Tenure PDFSarabeth Silver MacapagaoNo ratings yet
- Estimated Difference in Subsidies Between The AHCA and The ACA by Zip CodeDocument21 pagesEstimated Difference in Subsidies Between The AHCA and The ACA by Zip CodeNorth Star Policy InstituteNo ratings yet
- Technical Manual: Weighing TerminalDocument122 pagesTechnical Manual: Weighing Terminalalfredo morenoNo ratings yet
- A 732 - A732M - 02 Equivalencia Ic AstmDocument8 pagesA 732 - A732M - 02 Equivalencia Ic AstmorivaldopenaNo ratings yet
- Republic of The Philippines National Police Commission Philippine National Police Balatan Municipal Police Station Balatan, Camarines SurDocument12 pagesRepublic of The Philippines National Police Commission Philippine National Police Balatan Municipal Police Station Balatan, Camarines SurRitcheLanuzgaDoctoleroNo ratings yet
- GOVT - Departments - Contact - Details - MF-14-06-2021 UpdatedDocument32 pagesGOVT - Departments - Contact - Details - MF-14-06-2021 Updatedadf_raghuNo ratings yet
- ALKANES Quiz SheetDocument5 pagesALKANES Quiz Sheetnajifaahmed223No ratings yet
- Employees' State Insurance Corporation E-Pehchan Card: Insured Person: Insurance No.: Date of RegistrationDocument3 pagesEmployees' State Insurance Corporation E-Pehchan Card: Insured Person: Insurance No.: Date of RegistrationShankar KhuspeNo ratings yet
- Incident Report TemplateDocument3 pagesIncident Report Templateapi-412577219No ratings yet
- Bảng Tính Cột Áp QuạtDocument4 pagesBảng Tính Cột Áp QuạtNguyen PhamNo ratings yet
- Specification For Firewater Pump Package S 721v2020 08Document90 pagesSpecification For Firewater Pump Package S 721v2020 08Serge RINAUDONo ratings yet
- Student Admission FormDocument2 pagesStudent Admission FormOmmsai co2011No ratings yet
- Overview of Systemic Constellation WorkDocument15 pagesOverview of Systemic Constellation WorkKaren Carnabucci100% (2)
- EER WorksheetDocument3 pagesEER WorksheetMichael PoddubnyNo ratings yet
- Pre-Job Hazard AnalysisDocument4 pagesPre-Job Hazard AnalysisFiras HamanNo ratings yet
- Listado de Precios HADocument6 pagesListado de Precios HAgloria c.fernandezNo ratings yet
- 2008 Financial CrisisDocument34 pages2008 Financial CrisisJakeNo ratings yet
- Thuja's Effectiveness Against SmallpoxDocument361 pagesThuja's Effectiveness Against SmallpoxFrank NavaNo ratings yet
- Design Calculations For Snap Fit Joints in Plastic Parts Ticona PDFDocument30 pagesDesign Calculations For Snap Fit Joints in Plastic Parts Ticona PDFSuteu Ionel100% (1)
- Eastridge Golf Club, Inc V Eastridge Labor Union-SUPERDocument3 pagesEastridge Golf Club, Inc V Eastridge Labor Union-SUPERJames Evan I. ObnamiaNo ratings yet
- Pest Management Practices of Farmers in PambujanDocument13 pagesPest Management Practices of Farmers in PambujanLucille MoralesNo ratings yet
- AmoniacoDocument2 pagesAmoniacoAlejandra Morales0% (1)
- SS - 578 - 2012 - Use and Maintenance of Fire ExtinguishersDocument24 pagesSS - 578 - 2012 - Use and Maintenance of Fire Extinguishersrasanavaneethan100% (4)
- Intelli Trac X1Document2 pagesIntelli Trac X1nadjibnetNo ratings yet
- Supplementary Life Insurance - Enrolment FormDocument1 pageSupplementary Life Insurance - Enrolment FormjeevaNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Chemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastFrom EverandChemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastNo ratings yet
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- An Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksFrom EverandAn Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksRating: 5 out of 5 stars5/5 (1)
- Coating and Drying Defects: Troubleshooting Operating ProblemsFrom EverandCoating and Drying Defects: Troubleshooting Operating ProblemsRating: 5 out of 5 stars5/5 (1)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Gas-Liquid And Liquid-Liquid SeparatorsFrom EverandGas-Liquid And Liquid-Liquid SeparatorsRating: 3.5 out of 5 stars3.5/5 (3)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeRating: 4 out of 5 stars4/5 (9)
- Stuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldFrom EverandStuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldRating: 4 out of 5 stars4/5 (289)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- Chemical Elements Pocket Guide: Detailed Summary of the Periodic TableFrom EverandChemical Elements Pocket Guide: Detailed Summary of the Periodic TableNo ratings yet