Professional Documents
Culture Documents
DLP Ionss Science
Uploaded by
JOHNERROL CARCELLAROriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
DLP Ionss Science
Uploaded by
JOHNERROL CARCELLARCopyright:
Available Formats
lOMoARcPSD|20222059
DLP-ionss - science
Bachelor of Science and Information Technology (Bulacan State University)
Studocu is not sponsored or endorsed by any college or university
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
DETAILED LESSON PLAN IN SCIENCE - GRADE Nine
November 10, 2022 (Thursday) 5:00 P.M. - 6:00 P.M.
Grade 9 - Malacañang (Room )
I. OBJECTIVES
A. Content Standard
Forces that hold metals together.
B. Learning Competencies
MELCs: Explain how ions are formed
Code: (S9MTIIe-f-16)
II. CONTENT
A. Topic/Subject Matter:
IONS: How Are They Formed? (Quarter 2- Matter Module 3)
III. LEARNING RESOURCES
A. Instructional Materials:
Chalkboard, Chalk and Eraser
Laptop
PowerPoint Presentation
Printed pictures
• Visual Aids
Activity Sheets
White board
Magnets as electrons (dots)
B. References:
Learner’s Materials (LM): Pages
Teacher’s Guide (TG):
Curriculum Guide (CG):
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
Other References: YouTube:
Books
Webpage:
C. Skills
Enumerating
Participating
Collaborating
Identifying
Brainstorming
Communication Skills
Information and Communication Literacy
D. Science Concepts:
Chemical bond – is a term use to describe the attraction of atoms that are
combined together through sharing and transferring their valence electrons.
Ions – are atoms or groups of atoms that has positive or negative charge. Ions
are formed when an atom lose or gain its electrons. If metals were chemically
combined to nonmetals, metals would tend to lose electron while non-metals
gain electrons during chemical bonding to attain stability.
OCTET RULE – the tendency of atoms to prefer to have eight electrons in the
valence shell.
Cation – an atom that loses electrons becomes a positively charged ion.
Anion – an atom that gains electrons become negatively charged ion.
Lewis Electron Dot Structure (LEDS) – composed of the symbol of the
element and dots which represent the number of valence electrons of an atom
that can easily be determined through the group/family number in the Periodic
Table of Elements.
Valence Electrons – are electrons in the highest occupied energy level of an
atom.
E. Values Integration
Collaboration
Self-respect
Respectful
Resourcefulness
Cooperation
Open-mindedness
Adaptability
F. Teaching Strategies
Active Learning
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
Collaborative Teaching
Cooperative Learning
Technology-Based Classroom
Experiential Learning
Interactive and Participative Learning
Discussion and presentation
IV. LEARNING TASK/PROCEDURES
TEACHER’S ACTIVITY STUDENT’S ACTIVITY
PRELIMINARY ACTIVITIES
1. Prayer
Let us ask for the guidance of the Lord.
May I request everyone to stand up, (The chosen student will come in front and
(Student’s name) to lead the opening lead the opening prayer)
prayer. (The student leads the opening prayer)
Let us bow our head and feel the presence of
the Lord.
“Dear Lord and father of all. Thank you for
today. Thank you for ways in which you
provide for us, for your protection and love.
Guide us by your eternal light as we discover
more about the world around us. We ask all
this in the name of Jesus. Amen!.”
2. Greetings
Good Afternoon, Grade 9 Malacañang. Good Afternoon Teachers, Good Afternoon
visitors.
So how’s your day? Is it good so far?
We’re fine Ma’am!
3. Classroom Management
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
As your facilitator of learning, it is my goal
to give you an amazing learning
experience. To achieve it, your cooperation
is greatly needed by obeying the following
rules:
1. Using of gadgets is strictly prohibited
unless I allowed it. In case of
emergency, just ask properly. (The Students are listening attentively to the
2. Keep all the unnecessary things that are class rules)
not related to our subject.
3. Refrain from talking to your seatmates
and avoid making unnecessary
comments.
4. If you want to answer and you have
questions, just raise your hand.
Are we clear?
Yes Ma’am!
4. Checking of Attendance
Michelle do we have absentees for today?
(The students will tell if there is absentees or
none)
Okay, very good class!
5. Checking of Assignments
Do we have any assignment class?
None Ma’am.
A. REVIEWING PREVIOUS OR
PAST LESSON OR PRESENTING
THE NEW LESSON – ELICIT
Before we will discuss our next topic or
lesson for today. To recall your previous
lesson let us have an activity first. You’re
going to do is to determine the correct
answer that is written in the visual aids.
Students who want to answer just raise
your hands. Are we clear? Yes Ma’am.
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
For number 1, who wants to answer?
(Students from the class will raised their
hands to answer)
Okay (Student’s name), I see you want to
answer. The answer for number 1 ma’am is
Compound.
Okay (Student’s name) very good! How
(Students from the class will raised their
about for number 2? Who wants to
hands to answer)
answer?
Yes, (Student’s name)
The answer for number 2 ma’am is Ionic
Compound.
That is correct (Student’s name) very good.
(Students from the class will raised their
Let’s move to number 3.
hands to answer)
Yes, what about you (Student’s name)
Ma’am the answer for number 3 is Covalent
kindly answer number 3.
Compound
Okay, very good! For number 4 who wants
(Students from the class will raised their
to answer?
hands to answer)
Yes, (Student’s name) what is the answer?
The answer for number 4 ma’am is Water.
Okay, that is correct (Student’s name). For
(Students from the class will raised their
the last number who wants to answer?
hands to answer)
Yes. (Student’s name).
The answer for number 5 ma’am is Salt or
Table Salt.
Okay very good because of that give
yourselves an Bond Clap.
(Students will perform the Bond Clap)
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
B. ESTABLISHING A PURPOSE
FOR THE LESSON – ENGAGE
Before we proceed to our lesson let us
have a fun and exciting game. We are
going to play a “Mystery Box Games” We
all know to play this game we need a
participants every group.
To play the game, divide the students into
two teams and have them take turns
choosing a paper inside the mystery box.
Once the student gets the paper inside the
mystery box he/she can now guess the
answer, but if he/she did not know the
answer the other team will have a chance
to steal the points from them. Always
remember that in every correct answer is
equivalent to two points.
Are you ready class?
Yes, we are ready!
So we can now start.
(Students from every group will raised their
hands to join the game)
Congratulations class! Let’s give everyone
a Bond Clap.
(Students will perform the Bond Clap)
C. PRESENTING EXAMPLES /
INSTANCES OF THE NEW
LESSON
Now, what are your thoughts about our
recent activity? What did you feel? (The students will raise their hand to answer)
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
(The teacher will call someone from the
class)
We really enjoyed the activity, Ma’am!
That’s very good if you enjoyed the
activity. How about the others? What do
you think is our lesson for today? (The students will raise their hands)
(The teacher will call someone from the
class)
How ions are formed? Ma’am!
.
Very good! Today, we will discuss and
learn how ions are formed? I want all of
you to remain seated and all eyes are on
me and stay quiet.
D. DISCUSSING NEW CONCEPTS
AND PRACTICING NEW SKILLS
– EXPLORE
You may have heard about your mother
telling you to drink oral rehydration salts
when you are experiencing diarrhea to
replenish the ions your body may have
lost. Have you ever wondered what the
things that your body may have lost? Or
what are these oral rehydration salts
contain that prevent you from being
dehydrated every time you are having a
severe case of diarrhea?
Through the process of osmosis, the salts (Students listening attentively)
and sugars pull water into your
bloodstream and speed up rehydration.
Chemical bond – is a term use to describe
the attraction of atoms that are combined
together through sharing and transferring
their valence electrons.
Ions are atoms or groups of atoms that has (The students will raise their hand)
positive or negative charge.
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
Ions are formed when an atom lose or gain
its electrons.
(Someone from the class will read the
If metals were chemically combined to definition)
nonmetals, metals would tend to lose
electron while nonmetals gain electrons
during chemical bonding to attain stability.
OCTET RULE the tendency of atoms to
prefer to have eight electrons in the
valence shell.
Valence Electrons – are electrons in the
highest occupied energy level of an atom.
(Students listening attentively)
Cation – an atom that loses electrons
becomes a positively charged ion.
Anion – an atom that gains electrons
become negatively charged ion.
Lewis Electron Dot Structure (LEDS) –
composed of the symbol of the element
and dots which represent the number of
valence electrons of an atom that can easily
be determined through the group/family
number in the Periodic Table of Elements.
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
(Students listening attentively)
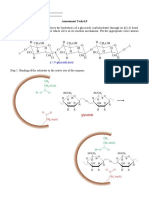
Lewis Electron Dot Structure of NaCl
Steps for Drawing a Lewis Diagram
Example:
Example:
Do you understand?
Yes ma’am!
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
Okay who wants to draw the Lewis dot (The students will raise their hand)
structure for BeFl2 and SO3
(The students answer may vary)
E. DISCUSSING NEW CONCEPTS
AND PRACTICING NEW SKILLS
So now, let’s exercise your knowledge.
May I request each to seat according to
your groupings, then group leaders will
come in front and get these envelopes
placed above the table. Inside this Yes ma’am!
envelopes are the activities you need to do
and present later on. Is that clear, 9-
Malacañang?
The following are the prepared activities (The students will raise their hand)
for every groups. (The picture below shows
the activity inside the envelopes.)
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
(The students answer may vary)
I will give each group 5 minutes to do their
activities, and then after that we will start
checking the answer of every group. Is the
instruction clear? (The representative of each group will check
the answer of the different groups)
F. FINDING PRACTICAL
APPLICATIONS OF CONCEPTS
AND SKILLS IN DAILY LIVING –
ELABORATE
After learning and gaining knowledge (Students will raise their hand to answer)
and information about how ions are
formed, what do you think is the
importance of its presence in our (The students answer may vary)
current times?
Yes Good answer!
(Students will raise their hand to answer)
Step 1: Select the center atom
How about in Lewis Electron Dot
Step 2: Put 2 electrons between the
Structure, what are the steps that we
atoms
need to follow?
Step 3: Complete the octet on outside
atoms
Step 4: Stable Lewis Structure
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
Okay, thank you! That is correct. I can see
that you now understand our lesson well
and you already achieved our main (Students will perform the Bond Clap)
objective for today and because of that
give yourselves an Bond Clap.
G. MAKING GENERALIZATIONS
AND ABSTRACTIONS ABOUT
THE LESSON - EXTEND
Now let’s have a quick summary of our
lesson. Let me pick a question on the box
and try to answer it, if you want to answer
kindly raise your hands.
(Students will raise their hand to answer)
(Pick questions randomly inside the box)
(Students will perform the Bond Clap)
Very good class! Let’s give them a Bond
Clap.
Is there any question or clarification, class? Yes ma’am.
Then if there are no more questions, are
you all ready to have your quiz for today?
I’ll give you 5 minutes to answer. (Students will start to answer the quiz)
Okay, time is up! Stop answering, class.
Please exchange papers with your
seatmate.
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
H. EVALUATING LEARNING/ EVALUATE
Choose the letter of the best answer. Write your answers on a separate sheet of paper.
1. Why do atoms tend to lose or gain electrons during chemical bonding?
A. to attain beauty
B. to attain stability
C. to become reactive
D. to attain malleability
2. What do we mean by stable configuration?
A. having 5 electrons in the outermost shell
B. having 6 electrons in the outermost shell
C. having 7 electrons in the outermost shell
D. having 8 electrons in the outermost shell
3. What type of element should bond together to form an ionic compound?
A. Metal and Nonmetal
B. Metal and Metalloids
C. Nonmetal and metalloid
D. Nonmetal and another Nonmetal
4. When a metal and nonmetal atom bond together to form an ionic compound they
become ions, what type of ion is formed by the metal atom?
A. Negative Ions
B. Neutral Ions
C. Positive Ions
D. Reactive Ions
5. When a metal and nonmetal atom bond together to form an ionic compound they
become ions, what type of ion is formed by the nonmetal atom?
A. Negative Ions
B. Neutral Ions
C. Positive Ions
D. Reactive Ions
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
6. What does it mean if K atom becomes K+ ions?
A. it has lost 1 electron
B. it has lost 2 electrons
C. it has gain 1 electron
D. it has gain 2 electrons
7. Which atom is most likely to form a 3+ ion?
A. Al B. Kr C. Li D. Si
8. Which is true about the elements in a particular group in the periodic table?
A. they have the same number of protons
B. they have the same number of electrons
C. they have the same number of energy levels
D. they have the same number of valence electrons
9. When writing the chemical formula for an ionic compound, which will you write first?
A. Positive ion
B. Negative ion
C. Charge of the ion
D. Subscript of the ion
10. How will you write the ion of Bromine that gains 1 electron during the process of
chemical bonding?
A. AB+1 B. B-1 C. Br+1 D. Br-1
Learning Agreements:
Complete the table below following the given example on number 1 (Lithium). Write your
answer on a separate sheet of paper.
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
lOMoARcPSD|20222059
16
Prepared by:
JUDITH R. GABAY
Practice Teacher
BSEd Sciences 4A
Approved by:
CARMEL M. CALIWLIW
Cooperating Teacher
16
Downloaded by JOHNERROL CARCELLAR (johnerrol.carcellar@ssu.edu.ph)
You might also like
- Detailed Lesson Plan: Mat107N - Number TheoryDocument20 pagesDetailed Lesson Plan: Mat107N - Number TheoryJoyline GarganeraNo ratings yet
- Local Demo 1 Midline TheoremDocument9 pagesLocal Demo 1 Midline Theoremjesibel roco100% (1)
- Basic Education Department Grade 9 St. Catherine of Siena DLPDocument8 pagesBasic Education Department Grade 9 St. Catherine of Siena DLPAljepric GalangNo ratings yet
- DLP in Science 3Document12 pagesDLP in Science 3Jefferson SisonNo ratings yet
- Final Demo Lesson Plan Olermo Bonifacio Jr. C. 1Document17 pagesFinal Demo Lesson Plan Olermo Bonifacio Jr. C. 1juniobendiegoNo ratings yet
- Local Demo 2Document11 pagesLocal Demo 2jesibel rocoNo ratings yet
- A Detailed Lesson Plan in Social Studies 6Document11 pagesA Detailed Lesson Plan in Social Studies 6Marisol Otida100% (1)
- Department of Education: San Jose Pili National High School-Tagbong ExtensionDocument6 pagesDepartment of Education: San Jose Pili National High School-Tagbong ExtensionMa. Theresa HabanaNo ratings yet
- Detailed Lesson Plan in TLE 9: Cronasia Foundation College IncDocument6 pagesDetailed Lesson Plan in TLE 9: Cronasia Foundation College Incnoy100% (1)
- Detailed LessonplanDocument7 pagesDetailed LessonplanCristine Kaye BandelanNo ratings yet
- Lesson Plan CoulombsDocument12 pagesLesson Plan CoulombsStephany Mae CanoyNo ratings yet
- Bagasbas F LPDocument19 pagesBagasbas F LPDan Marc BorjaNo ratings yet
- DLP in Science 3Document12 pagesDLP in Science 3Jefferson SisonNo ratings yet
- 7E Detailed Lesson PlanDocument11 pages7E Detailed Lesson Planjoeymatira0620No ratings yet
- Grade 8 Detailed Lesson Plan in MathematicsDocument11 pagesGrade 8 Detailed Lesson Plan in MathematicsJoemel SarmientoNo ratings yet
- Velarde DLP 3Document10 pagesVelarde DLP 3Jhoan Cinco VelardeNo ratings yet
- A DLP SCIENCE 10 Electromagnetic Spectrum 1Document9 pagesA DLP SCIENCE 10 Electromagnetic Spectrum 1Blessing Shielo VicenteNo ratings yet
- Ldetailed LP in PRO ED 7Document14 pagesLdetailed LP in PRO ED 7Mayare, Angelyn- BEED IINo ratings yet
- Group 4 Dlp..forms of EnergyDocument12 pagesGroup 4 Dlp..forms of EnergybangalitsabbfNo ratings yet
- SMPLDLPDocument11 pagesSMPLDLPdrky2mms5qNo ratings yet
- Cronasia Foundation College Inc.: TLE - IAEI7/8UT-0a-1Document9 pagesCronasia Foundation College Inc.: TLE - IAEI7/8UT-0a-1noyNo ratings yet
- Multimodal Texts DLPDocument30 pagesMultimodal Texts DLPveronica dumanopNo ratings yet
- For Stephany MaeDocument17 pagesFor Stephany MaeStephany Mae CanoyNo ratings yet
- DLP - Dessert Final DemoDocument13 pagesDLP - Dessert Final DemoMarj P. Malalay GablinezNo ratings yet
- QUANTUM MODEL OF ATOM-Lesson-PlanDocument13 pagesQUANTUM MODEL OF ATOM-Lesson-PlanVANESSA PEDRONo ratings yet
- 4a's DETAILED LESSON PLAN IN SCIENCE 5, 3RD QUARTERDocument9 pages4a's DETAILED LESSON PLAN IN SCIENCE 5, 3RD QUARTERGwyneth Queen GalvadoresNo ratings yet
- Detailed Lesson Plan in English 2Document12 pagesDetailed Lesson Plan in English 2Ing ridNo ratings yet
- Revised Lesson PlanDocument5 pagesRevised Lesson PlanChrisna Ian Lou EneroNo ratings yet
- Morata Dlpscience4 FourthquarterDocument9 pagesMorata Dlpscience4 FourthquarterJustine MorataNo ratings yet
- Cedillo LP DemoDocument12 pagesCedillo LP DemoAngel Nicole ArceloNo ratings yet
- FS LESSON PLAN (AutoRecovered)Document10 pagesFS LESSON PLAN (AutoRecovered)Rosemarie GaringNo ratings yet
- LP Grade 8 MolaveDocument19 pagesLP Grade 8 MolaveBlessing Shielo VicenteNo ratings yet
- Hangad LP Q4Document9 pagesHangad LP Q4Ramil HangadNo ratings yet
- DLP EnglishDocument14 pagesDLP EnglishKristine Joy PerezNo ratings yet
- DLP-Ma'am Totao-AisaroyceDocument12 pagesDLP-Ma'am Totao-AisaroyceAisa Royce DemolNo ratings yet
- Week 1 G10Document10 pagesWeek 1 G10Richel CastilloNo ratings yet
- Toaz - Info A Detailed Lesson Plan in Social Studies 6 PRDocument11 pagesToaz - Info A Detailed Lesson Plan in Social Studies 6 PRCherine EmpuertoNo ratings yet
- A Detailed Lesson Plan in Science 6Document11 pagesA Detailed Lesson Plan in Science 6Angel Mae H. Solamin100% (1)
- CO1 G8 2023 Deped ApplicantDocument7 pagesCO1 G8 2023 Deped ApplicantClarisse De GuzmanNo ratings yet
- A Detailed Lesson Plan in English 10Document8 pagesA Detailed Lesson Plan in English 10Jennecil Tigao AmargaNo ratings yet
- LeoDocument13 pagesLeoleonardoalbor05No ratings yet
- Edeng - Detailed Lesson Plan (Direct Method)Document10 pagesEdeng - Detailed Lesson Plan (Direct Method)Miralona RelevoNo ratings yet
- Final-DLP Behavior of GasesDocument17 pagesFinal-DLP Behavior of GasesjosellsulfelixNo ratings yet
- Nueva Ecija University of Science and Technology: - ObjectivesDocument6 pagesNueva Ecija University of Science and Technology: - ObjectivesEvangeline Cruz OrtizNo ratings yet
- LESSON PLAN For DIGESTIVE SYSTEMDocument7 pagesLESSON PLAN For DIGESTIVE SYSTEMela ravenaNo ratings yet
- Lesson Plan in Englih 6Document8 pagesLesson Plan in Englih 6Judith Lyka Dela CruzNo ratings yet
- Final Demo LPDocument16 pagesFinal Demo LPalexandraroxas777No ratings yet
- Sample Detailed Lesson Plan in English For Teaching DemonstrationDocument7 pagesSample Detailed Lesson Plan in English For Teaching DemonstrationWenna CellarNo ratings yet
- LESSON PLAN - Group 2Document3 pagesLESSON PLAN - Group 2Noel Calvo MacarineNo ratings yet
- q2w2 Eals DLP Detailed Lesson Plan in Earth and Life ScienceDocument9 pagesq2w2 Eals DLP Detailed Lesson Plan in Earth and Life ScienceMarfe MontelibanoNo ratings yet
- Pinagtonulan Integrated National High SchoolDocument8 pagesPinagtonulan Integrated National High SchoolAngela CatainaNo ratings yet
- DLP ScienceDocument11 pagesDLP ScienceCarrie Macanip MacarayoNo ratings yet
- Trends and Issues Nov. 15Document16 pagesTrends and Issues Nov. 15Hazeline SobremonteNo ratings yet
- 4a's Detailed Lesson Plan For Mam Jewel WordDocument5 pages4a's Detailed Lesson Plan For Mam Jewel WordMarie mel ElumbaNo ratings yet
- San Matias National High School: Detailed Lesson Plan I. ObjectiveDocument11 pagesSan Matias National High School: Detailed Lesson Plan I. ObjectiveAdrian B. OrtegaNo ratings yet
- JackandtheBeanstalk PDFDocument6 pagesJackandtheBeanstalk PDFLovely HerreraNo ratings yet
- Final DemoDocument17 pagesFinal DemoJerico SimNo ratings yet
- DLP For Eng8 Q4-M2Document12 pagesDLP For Eng8 Q4-M2Rachelle Marie AlejandroNo ratings yet
- Detailed Lesson in Practical Research 1 Data Analysisa4 PDF FreeDocument7 pagesDetailed Lesson in Practical Research 1 Data Analysisa4 PDF FreeJenn BicoNo ratings yet
- Conversational English for a Global Society (Intermediate Level)From EverandConversational English for a Global Society (Intermediate Level)No ratings yet
- AssessmentDocument13 pagesAssessmentJOHNERROL CARCELLARNo ratings yet
- PP John ObjectiveDocument1 pagePP John ObjectiveJOHNERROL CARCELLARNo ratings yet
- Sci9 Q4 Mod8.2Document7 pagesSci9 Q4 Mod8.2JOHNERROL CARCELLARNo ratings yet
- QuizDocument6 pagesQuizJOHNERROL CARCELLARNo ratings yet
- Third Mean and Mps Word GraphDocument2 pagesThird Mean and Mps Word GraphJOHNERROL CARCELLARNo ratings yet
- Tingog Smart 2024Document156 pagesTingog Smart 2024JOHNERROL CARCELLARNo ratings yet
- Questions For ExaminationDocument4 pagesQuestions For ExaminationJOHNERROL CARCELLARNo ratings yet
- DLP Carcellar 1Document14 pagesDLP Carcellar 1JOHNERROL CARCELLARNo ratings yet
- Projectile MotionDocument11 pagesProjectile MotionJOHNERROL CARCELLARNo ratings yet
- In Campus Fs 2 Summary of RatingsDocument2 pagesIn Campus Fs 2 Summary of RatingsJOHNERROL CARCELLARNo ratings yet
- DLP CarcellarDocument8 pagesDLP CarcellarJOHNERROL CARCELLARNo ratings yet
- SSU OVPAA FR 018 Course Syllabus Sci6Document11 pagesSSU OVPAA FR 018 Course Syllabus Sci6JOHNERROL CARCELLARNo ratings yet
- Arrangement of Star in Group-1Document11 pagesArrangement of Star in Group-1JOHNERROL CARCELLARNo ratings yet
- Rationalization of The 1st Pretest in CE1Document51 pagesRationalization of The 1st Pretest in CE1JOHNERROL CARCELLARNo ratings yet
- Enzyme MechanismDocument3 pagesEnzyme MechanismJOHNERROL CARCELLARNo ratings yet
- Curriculum Development Models and Designs AutosavedDocument45 pagesCurriculum Development Models and Designs AutosavedJOHNERROL CARCELLARNo ratings yet
- Prof Ed 8 LP4 2Document13 pagesProf Ed 8 LP4 2JOHNERROL CARCELLARNo ratings yet
- Organizational LeadershipDocument26 pagesOrganizational LeadershipJOHNERROL CARCELLARNo ratings yet
- PBL InstrumentDocument1 pagePBL InstrumentJOHNERROL CARCELLARNo ratings yet
- Great Books 1001Document7 pagesGreat Books 1001JOHNERROL CARCELLARNo ratings yet
- Learning Packet 1 Unit 1 Prof - Ed.7Document15 pagesLearning Packet 1 Unit 1 Prof - Ed.7JOHNERROL CARCELLARNo ratings yet
- Comparative Analysis North Korea Jessa AngelicaDocument6 pagesComparative Analysis North Korea Jessa AngelicaJOHNERROL CARCELLARNo ratings yet
- Group 1 Presentors Media and Digital LiteracyDocument28 pagesGroup 1 Presentors Media and Digital LiteracyJOHNERROL CARCELLARNo ratings yet
- LP 4 FINAL Waves and OpticsAutosaved Copy 1Document15 pagesLP 4 FINAL Waves and OpticsAutosaved Copy 1JOHNERROL CARCELLARNo ratings yet
- Prof Ed 8 LP3 MergedDocument29 pagesProf Ed 8 LP3 MergedJOHNERROL CARCELLARNo ratings yet
- Weatherforecasting RevisedDocument4 pagesWeatherforecasting RevisedJOHNERROL CARCELLARNo ratings yet
- REFLECTIONDocument2 pagesREFLECTIONJOHNERROL CARCELLARNo ratings yet
- LP2 SCI-12 MICROBIO LABORATORY-fINALDocument21 pagesLP2 SCI-12 MICROBIO LABORATORY-fINALJOHNERROL CARCELLARNo ratings yet
- Lp4 Sci 13 Virology StudentsDocument15 pagesLp4 Sci 13 Virology StudentsJOHNERROL CARCELLARNo ratings yet
- LP 5 Parasitology 2021Document15 pagesLP 5 Parasitology 2021JOHNERROL CARCELLARNo ratings yet
- Polymer Additives...Document37 pagesPolymer Additives...Enaye MajiriNo ratings yet
- Distillation Through Fractional ColumnDocument6 pagesDistillation Through Fractional ColumnM. Shehryar KhanNo ratings yet
- Half-Life CalculationDocument24 pagesHalf-Life CalculationAllNo ratings yet
- Multiple Choice Questions: Question Bank Class: Xii, Chemistry Unit 4: Haloalknaes & HaloarenesDocument27 pagesMultiple Choice Questions: Question Bank Class: Xii, Chemistry Unit 4: Haloalknaes & HaloarenesAkshita BoroNo ratings yet
- St. Mark's Sr. Sec. Public School, Janakpuri PA-II Exam Class XII Subject: ChemistryDocument22 pagesSt. Mark's Sr. Sec. Public School, Janakpuri PA-II Exam Class XII Subject: ChemistryPp PpNo ratings yet
- Lee EFSDocument323 pagesLee EFSKamesh JaiNo ratings yet
- Molecular Geometry and Bonding TheoriesDocument5 pagesMolecular Geometry and Bonding TheoriesPineraserNo ratings yet
- GNS311 SUMMARY (The New Edition) by ASF UnilorinDocument32 pagesGNS311 SUMMARY (The New Edition) by ASF Unilorinmo sopeNo ratings yet
- Fluorescent Magnetic TestingDocument29 pagesFluorescent Magnetic TestingAlzaki Abdullah100% (1)
- 3.classification of ElementsDocument18 pages3.classification of ElementsMUHAMMAD YASEENNo ratings yet
- Naming and Formula Practice QuizDocument2 pagesNaming and Formula Practice QuizSharesse Joy GumalalNo ratings yet
- MM-18 - Bilge Separator - OPERATION MANUALDocument24 pagesMM-18 - Bilge Separator - OPERATION MANUALKyaw Swar Latt100% (2)
- Chem 18.1 Lab Report 1Document7 pagesChem 18.1 Lab Report 1Rhic Vincent MorenoNo ratings yet
- Titration Level 1: Krizzi AimsDocument4 pagesTitration Level 1: Krizzi AimsKrizzi Dizon GarciaNo ratings yet
- Astm G 35 - 98 - RZM1LTK4Document3 pagesAstm G 35 - 98 - RZM1LTK4Cordova RaphaelNo ratings yet
- GC Fatty Acid Methyl EstersDocument11 pagesGC Fatty Acid Methyl EstersReem MohamedNo ratings yet
- Geosynthetics - Geocells - Specification: Indian StandardDocument20 pagesGeosynthetics - Geocells - Specification: Indian StandardAshish WaliaNo ratings yet
- Chemistry Notes (Electrolysis)Document2 pagesChemistry Notes (Electrolysis)Teo Jia Ming Nickolas100% (3)
- REVIEWDocument9 pagesREVIEWRaviraj MalaniNo ratings yet
- Carbon Disulphide PlantDocument8 pagesCarbon Disulphide PlantDipanjanSarkarNo ratings yet
- Mass Transfer Operations 2020Document325 pagesMass Transfer Operations 2020EJ TanNo ratings yet
- Ebook Chemistry 6Th Edition Mcmurry Test Bank Full Chapter PDFDocument67 pagesEbook Chemistry 6Th Edition Mcmurry Test Bank Full Chapter PDFricinussquabash.46iz9100% (10)
- Lecture - Slides - 2.3 The Two-Terminal MOS Structure - General AnalysisDocument6 pagesLecture - Slides - 2.3 The Two-Terminal MOS Structure - General AnalysisDharmendra Mani VarmaNo ratings yet
- C83IA006EN C ApplReport Cleaning Recs AlcolyzerDocument2 pagesC83IA006EN C ApplReport Cleaning Recs AlcolyzerXavier ArévaloNo ratings yet
- C 1305 - 00 - QzezmduDocument3 pagesC 1305 - 00 - Qzezmdumercab15No ratings yet
- LiOH Con AlcoholDocument6 pagesLiOH Con Alcoholm_condorettyNo ratings yet
- LiposomesDocument71 pagesLiposomesDr. Aliha AkhtarNo ratings yet
- AASHTO T 272 - Standard Method of Test For One-Point Method For Determining Maximum DryDocument5 pagesAASHTO T 272 - Standard Method of Test For One-Point Method For Determining Maximum DryYuri ValenciaNo ratings yet
- Sample Exp 6 CHM 477Document11 pagesSample Exp 6 CHM 477ommy madinaNo ratings yet
- Teacher Guide: Mystery Powder Analysis: Learning ObjectivesDocument3 pagesTeacher Guide: Mystery Powder Analysis: Learning Objectiveswilliam gomezNo ratings yet