Professional Documents
Culture Documents
1c States of Matter ANSWERS
Uploaded by
Karina LeungOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
1c States of Matter ANSWERS
Uploaded by
Karina LeungCopyright:
Available Formats
Island School Chemistry ANSWERS 1c
States of Matter
Aim: 1.1 understand the arrangement, movement and energy of the particles in each of the
three states of matter: solid, liquid and gas
1.2 understand how the interconversion of solids, liquids and gases are achieved and recall the
names used for these interconversions
Questions:
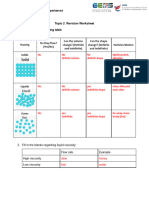
1. Complete the following table to summarise the properties of solids, liquids and gases
(some has been done for you)
State Solid Liquid Gas
Arrangement Very close together Close together .Far apart
Regular arrangement Random arrangement Random arrangement
Motion Vibrate about a fixed Slip and slide over each Move quickly in all
position other directions
Properties Definite shape Take shape of the Spread out to fill
(shape, volume & container available volume
compressibility)
Definite volume Volume depends on the
Definite volume container
Not compressible Not compressible Compressible
Model of
particles
2. Which of the 3 states of matter has the most energy? ......................gas............... (1)
3. Solids, liquids and gases can be interconverted by either heating or cooling. Complete
the following diagram by adding the appropriate name for the interconversion on the
appropriate arrows. (4)
Melting evaporating/boiling
Solid liquid gas
Freezing condensing
4. What is the difference between evaporation and boiling? .......evaporation can occur at
any temperature but boiling only occurs at one temperature when all the molecules
have sufficient energy to escape........ (2)
You might also like
- States of MatterDocument4 pagesStates of Matterahmed5030 ahmed5030100% (2)
- States of Matter Worksheet For STEDocument2 pagesStates of Matter Worksheet For STECaryl Ann C. Sernadilla100% (1)
- 1d States of Matter (2017)Document1 page1d States of Matter (2017)Karina LeungNo ratings yet
- MAT T Er in Our SurroundingDocument8 pagesMAT T Er in Our SurroundingBharathNo ratings yet
- Third Form Packet WorkDocument19 pagesThird Form Packet WorkLizbeth Chi100% (1)
- Understanding Kinetic Particle TheoryDocument50 pagesUnderstanding Kinetic Particle TheoryozmanNo ratings yet
- Chem 1Document58 pagesChem 1Heba HebaNo ratings yet
- LEARNING ACTIVITY SHEET 1 - Science 10Document3 pagesLEARNING ACTIVITY SHEET 1 - Science 10cherrymaeregalario2001No ratings yet
- Particulate Nature of MatterDocument36 pagesParticulate Nature of MatterAreejNo ratings yet
- Topic 2 Kinetic Particle Theory AnswersDocument6 pagesTopic 2 Kinetic Particle Theory AnswersKaixin HuangNo ratings yet
- States of MatterDocument3 pagesStates of Mattermohammed mahdyNo ratings yet
- CombinepdfDocument14 pagesCombinepdfIzzah ImranNo ratings yet
- Matter and Kinetic TheoryDocument4 pagesMatter and Kinetic TheoryAlex noslenNo ratings yet
- Matter in Our SurroundingsDocument11 pagesMatter in Our SurroundingsSandeep Kumar VRNo ratings yet
- 2.3 Changing States & Water CycleDocument65 pages2.3 Changing States & Water CycleJohn Michael DitchonNo ratings yet
- Class 9-Chemistry CW-1Document7 pagesClass 9-Chemistry CW-1aadithya.v.5502.sssmscNo ratings yet
- Properties of States of MatterDocument32 pagesProperties of States of MatterKuya Tiong TutorialsNo ratings yet
- 3 1Document90 pages3 1Joy MercadoNo ratings yet
- Core Notes by Dr. Maha FariedDocument122 pagesCore Notes by Dr. Maha Fariedmmkhst005No ratings yet
- Chapter 1 - Matter in Our SurroundingsDocument8 pagesChapter 1 - Matter in Our SurroundingsJitendra ChandelNo ratings yet
- Qa-Matter in Our SurroundingsDocument17 pagesQa-Matter in Our SurroundingsSubhajeet PaulNo ratings yet
- Chemistry 10 To 12 Notes 2nd EdDocument321 pagesChemistry 10 To 12 Notes 2nd EdXavierNo ratings yet
- Chemistry 10 To 12 Notes PDFDocument321 pagesChemistry 10 To 12 Notes PDFMoses NjobvuNo ratings yet
- 0 071711 G6End-Term1SummaryDocument19 pages0 071711 G6End-Term1Summaryziadlaharthi1No ratings yet
- Particulate Nature of Matter, Unit1Document15 pagesParticulate Nature of Matter, Unit1Keeertththana SaravananNo ratings yet
- Getting To Know Gases: Worksheet 4.1Document2 pagesGetting To Know Gases: Worksheet 4.1yunohahageNo ratings yet
- LN1 Chem NotesDocument4 pagesLN1 Chem Notesridhimarani16207No ratings yet
- SCIENCE-10 Q4 MOD1 Behavior-of-Gases BookletDocument12 pagesSCIENCE-10 Q4 MOD1 Behavior-of-Gases BookletRetep Aren50% (2)
- CHEM-Chapter 2 - State of MatterDocument13 pagesCHEM-Chapter 2 - State of Mattersecag45630No ratings yet
- Chemistry Particles - (g7)Document5 pagesChemistry Particles - (g7)Abdelrahman mohammedNo ratings yet
- Ram Gelo de Luna Haway - ACTIVITY NO. 1 (General Chemistry)Document5 pagesRam Gelo de Luna Haway - ACTIVITY NO. 1 (General Chemistry)Ram Gelo HawayNo ratings yet
- F1 Chapter 5 MatterDocument13 pagesF1 Chapter 5 Matteralya sophiaNo ratings yet
- CBSE Class 9 Science Revision Notes Chapter - 1 Matter in Our SurroundingsDocument68 pagesCBSE Class 9 Science Revision Notes Chapter - 1 Matter in Our SurroundingsParesh RanjanNo ratings yet
- Ncert Solutions For Class 9 Science Jan14 Chapter 1 Matter in Our SurroundingsDocument9 pagesNcert Solutions For Class 9 Science Jan14 Chapter 1 Matter in Our SurroundingsRINA MandalNo ratings yet
- States of Matter Solids Liquids and GasesDocument3 pagesStates of Matter Solids Liquids and GasesMatipa DembureNo ratings yet
- CHAPTER 1 Matter, MeasurementsDocument23 pagesCHAPTER 1 Matter, MeasurementsRusher SigueNo ratings yet
- Inbound 6566810336175603384Document41 pagesInbound 6566810336175603384jheniercapsNo ratings yet
- States of Matter SummaryDocument1 pageStates of Matter SummarySAMI DHAOUINo ratings yet
- Chapter 1 MatterDocument79 pagesChapter 1 MatterdwyquishNo ratings yet
- CSO Olympiad Book For Class 9Document10 pagesCSO Olympiad Book For Class 9Malith MadushanNo ratings yet
- Chem NotesDocument172 pagesChem NotesTshegofatso LegadikoNo ratings yet
- 01 States of MatterDocument3 pages01 States of MatterbadhriNo ratings yet
- NeverthelessDocument12 pagesNeverthelessravi chandranNo ratings yet
- Reviewer in Science 8 3RD QuarterDocument5 pagesReviewer in Science 8 3RD QuarterJillianNo ratings yet
- MATTERDocument9 pagesMATTERslathakamatchiNo ratings yet
- Ch. 1 Particulate Nature of Matter 2023 Year 10Document10 pagesCh. 1 Particulate Nature of Matter 2023 Year 10sarah awadNo ratings yet
- (CSEC Chemistry) Section A Notes and ESQsDocument191 pages(CSEC Chemistry) Section A Notes and ESQsNathaniel WhyteNo ratings yet
- UH Science 33Document4 pagesUH Science 33yuniNo ratings yet
- Matter in Our Surroundings - NotesDocument18 pagesMatter in Our Surroundings - NotesRajveer KaushalNo ratings yet
- Chemistry 0620 NotesDocument192 pagesChemistry 0620 Notesmohammed mahdyNo ratings yet
- CT - 3Document45 pagesCT - 3Snehal BhasinNo ratings yet
- Matter in Our SurroundingsDocument26 pagesMatter in Our SurroundingsULTRA BOSSNo ratings yet
- 09 Science Notes Ch01 Matter in Our SurroundingsDocument9 pages09 Science Notes Ch01 Matter in Our SurroundingsAmbika RamakrishnanNo ratings yet
- Answer KeyT12L2 - Change of State Final Exam Revision WorksheetDocument4 pagesAnswer KeyT12L2 - Change of State Final Exam Revision WorksheetmeexehahahaNo ratings yet
- 09 Science Notes Ch01 Matter in Our SurroundingsDocument6 pages09 Science Notes Ch01 Matter in Our SurroundingsFiraz CompNo ratings yet
- Chapter 5 Matter in NatureDocument1 pageChapter 5 Matter in NatureYuhannNo ratings yet
- Children Encyclopedia Chemistry: The World of KnowledgeFrom EverandChildren Encyclopedia Chemistry: The World of KnowledgeRating: 5 out of 5 stars5/5 (3)
- 1b Glossary Particles and Bonding (2017)Document2 pages1b Glossary Particles and Bonding (2017)Karina LeungNo ratings yet
- 4b ANSWERS Noble Gases (2017)Document1 page4b ANSWERS Noble Gases (2017)Karina LeungNo ratings yet
- Periodic TableDocument1 pagePeriodic TableSubbash EkambaramNo ratings yet
- 4a Periodic PatternsDocument2 pages4a Periodic PatternsKarina LeungNo ratings yet
- 3c More EquationsDocument2 pages3c More EquationsKarina LeungNo ratings yet
- 3b Balancing Equations CGPDocument1 page3b Balancing Equations CGPKarina LeungNo ratings yet
- 2c ANSWERS Balancing Equations (2017)Document1 page2c ANSWERS Balancing Equations (2017)Karina LeungNo ratings yet