Professional Documents
Culture Documents
Gaseous State and Thermodynamics - Level 3 - Solutions
Gaseous State and Thermodynamics - Level 3 - Solutions
Uploaded by
demolition squadOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Gaseous State and Thermodynamics - Level 3 - Solutions
Gaseous State and Thermodynamics - Level 3 - Solutions
Uploaded by
demolition squadCopyright:
Available Formats
Level - 3 JEE Advanced++ Pattern/Solutions
101. P1 P2 P3
If T0 T1 Then on an average, gas loses energy to the wall i.e. upon energy collision, gas molecules returns
with a lesser speed than the initial speed. Hence change in momentum is lesser. Hence lower pressure when
T T0 .
102. (i) 1 105 cm 3 (ii) 0.2 mm.
PV
N where N is number of molecules.
kT
Putting V 1cm 3 10 6 m 3
4 10 15 1.01 105 10 6
N 105
1.38 10 23 300
V
N
r3
2
2V 2 10 6
r3
N 105
r 3 2 10 11
r 0.27 mm
103. 1 atm
p pmolecule
F N
t t
where pmolecule is change in momentum per molecules and N is number of molecules colliding in time t
Now, pmolecule 2mv cos and N n A cos
F n ( A cos )[m (2v cos )]
F
2nmv 2 cos2
A
P 2nmv 2 cos2
104. 6.3 1012 rad / sec
Di-atomic molecule will have 2 rotational degree of freedom
1 1 2 2kT
2 k T I rms ; rms
2 2 I
105. 60 cm
After placing additional mass piston of cylinder A will come at the bottom and entire gas will be in cylinder B.
Initially :
Let pressure of gas is P.
m A.g mB .g
Since force on both pistons is balanced, P
AA AB
Gaseous State and Thermodynamics 87 Workbook – 3 | Solutions
AB
2
AA
P .( A A h ) P ( A B .h )
n1 ,n 2
RT RT
P ( A B h )
Finally (n1 n 2 )
RT
( A A AB )h A B h
1
h h 1 60cm
2
106. 2.6 gm
Final pressure will become 1 atm. Hence volume of bag will also become 2 ltr. Buoyant force on bag due to
air
B air .V . g
B 1.29 2 10 3 g
B
Additional mass is to be placed to balance buoyancy force, m 2.6 10 3kg
g
107. a T, b V, x 3, y 2, z 1
Straight line passing through origin for VT graph is isobaric.
Isothermal process on a PV graph looks like zy part on 2nd graph.
Hence only third option fits.
17 7
108. T T0 , V V0
15 10
Initially let n is the total no. moles in both containers.
2P0V0 P0V0 3P0V0
n
RT0 RT0 RT0
Finally
2P0V 2 P0V0
n
RT RT
3P0V0 2P0V 2 P0V0
. . . . . (i)
RT0 RT RT
Applying first law of thermodynamics on both cylinders together as a system
Q U W
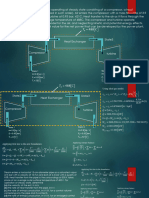
0 nCV ( T T0 ) 2P0 (V V0 )
3P0V0 3 R
0 (T T0 ) 2 P0 (V V0 )
RT0 2
By solving (1) and (2)
17 7
T T0 & V V0
15 10
109. RT/Mg
Mg
.y
In an isothermal atmosphere variation of density with height is given by 0e RT
Gaseous State and Thermodynamics 88 Workbook – 3 | Solutions
Mg
Let K . Then, P P0e Ky
RT
For centre of mass.
ydm
ycm 0
dm
0
Denominator : D Ady
P0 A e Kydy
0
P0 A
. . . . .(i)
K
Numerator : N P0 A y . e Kydy
0
Integrating by parts
Ky
e Ky e
N P0 A y
K 0
k
dy
0
P0 A
Using L’ Hospital’s rule for 1st term, N
K2
So,
P0 A
2 1 RT
ycm K
P0 A K Mg
K
Mg / aRT0 Mg / aRT0
110. (i) P P0 1 ah ; h 1/ a (ii) P P0 1 ah
dP gdh
PM
dP gdh [Using Ideal gas equation : PM RT
RT
dP Mg dh
P R T
P h
dP Mg dh
(i)
P
R T0(1 ah )
P0 0
Mg
P P0 (a ah ) aRT0
P h Mg
dp Mg dh aRT0
(ii)
P
R T0(1 ah ) ; P P0 (1 ah )
P0 0
Gaseous State and Thermodynamics 89 Workbook – 3 | Solutions
2 2
111. P P0e M r / 2RT
Net force on element towards axis of rotation, F AdP (dm )2 x
AdP (Adx )(2 x )
dP 2 x dx
PM 2
dP xdx
RT
P r
dP M 2
P
RT xdx
P0 0
P M 2r 2
n
P0 2RT
M 2r 2
P P0 e 2RT
p0 A mg
112.
2g
Initially, force on piston is balanced
PA mg P0 A
mg P0 A
P
A
Finally to lift the cylinder, net force on the bottom of the cylinder should be in upwards direction
P A Mg P0 A
P
Where P
2
PA
Mg P0 A
2
PA
Mg P0 A
2
mg P0 A
Mg P0 A
2 2
P0 A mg
Mg
2 2
P0 A mg
M
2g
5
113. V0
8
dP P
For adiabatic curve, Slope =
dV V
P0
Equation for the given P – V curve, which is a straight line: P V P0 . . . . (i)
V0
Gaseous State and Thermodynamics 90 Workbook – 3 | Solutions
P0
So, slope of this line =
V0
When process turns from endothermic to exothermic at that point dQ 0 , i.e. an adiabatic curve will touch
the given line at that point. At the point of touching, slope of the adiabatic curve should be equal to the slope
of the given line.
P0 P
. . . . .(ii)
V0 V
5 5
Solving (i) and (ii) and using , V V0
3 8
mghR
114. T0 1
M p0 p VC V
No of moles of air inside ball
(P0 P )V
n
RT0
When ball hits the ground
1
mv 2 mgh
2
Kinetic energy will convert to internal energy
1
mv 2 nCv T
2
(P0 P )
mgh Cv V (T T0 )
RT0
mghR
T T0 1
( P0 P ) VCv
3nr0 R
115. v 5mm / s
8kl 0
When the piston is stationary, all the beat goes into increasing temperature. So,
dQ du f dT
nR
dt dt 2 dt
f
nRr0 . . . . .(i)
2
When the piston is moving, at the instant its speed is V0
dw
rate of work done by gas PAV0
dt
dU f dT
Also, nR
dt 2 dt
By 1st law,
d du dw
dt dt dt
f f dT
From (i) nRr0 nR PAV0 . . . . (ii)
2 2 dt
Also, assuming quasistatic process, force on piston is balanced
Gaseous State and Thermodynamics 91 Workbook – 3 | Solutions
So, K ( 0 r ) PA
Using PV nRT ,( 0 x ) A nRT
k
Solving, T ( 0 x )2 . . . . (iii)
nR
dT 2K dx
Differentiating : ( 0 x ) .
dt nR dt
At x = 0, after a lot of solving, we get
3r0nR
V0
8K 0
116. Q1 Q 2
By 1st law thermodynamics for 1 2 Q1 U1 W1
( P P ) (V V )
Where W1 0 1 1 0
2
As the gas goes from 1 to 3, (Points 2, 3 lie on the same
isotherm), the following relations are fulfilled :
Q2 U 2 W2
( P0 P2 ) (V2 V0 )
Where W2
2
For isotherm : U1 U 2
In order to find out in which process Q is larger, we need to compare W1 and W2 .
( P0 P1 ) (V1 V0 ) ( P0 P2 ) (V2 V0 )
W1 W2
2 2
( P0V1 P0V2 ) ( P2V0 P1V0 )
0
2
Since, P0V1 P0V2 and P2V0 P1 V0 Q1 Q 2
C
t
117. P P0 e V
Let the instantaneous pressure and volume of the gas be P and V respectively. Since the process is
isothermal,
PV constant
Taking time derivative
dV dP
P V . . . . (i)
dt dt
dV
It is given that the rate of evacuation: C
dt
Here, note carefully that as soon as some small amount of gas of volume dV is taken out of the container,
the remaining gas has exactly dV volume more to occupy. So, since we are applying the equations for the
dV
thermodynamic process on the gas remaining in the container, the volume is increasing, and hence is
dt
positive.
Using this in equation (i),
PCdt VdP
Gaseous State and Thermodynamics 92 Workbook – 3 | Solutions
t P
dP
C
dt V P
0 P0
C
t
Ct V n (P / P0 ) P P0e V
118. T (P0 S mg) 0.9 K
R
Let the area of upper and lower pistons be A1 and A2 . Then, balancing forces,
P0 A1 PA2 mg PA1 P0 A2
P ( A1 A2 ) mg P0 ( A1 A2 )
mg
P P0
A1 A2
Pressure of gas remains constant as the piston moves up or down. So, heating the gas results in upward
motion of pistons which increases volume while keeping pressure constant. Hence, this is an isobaric
process.
P V nR T
mg ( A1 A2 )
T P0
( A1 A2 ) nR
Put n 1 and A1 A2 S
T ( P0 S mg )
R
3m 2 R
119.
2m1
Suppose that piston is displaced rightward by some distance. As a result, the system (1 + 2) gains some
energy in the form of work. Therefore, temperature of system increases.
Note carefully that temperature of both parts will remain same at all instants as the partition dividing them
is conducting. So heat flows from the left compartment to the right. Thus, the left compartment is losing heat
and yet its temperature is rising.
Let dQ be heat transferred from 1 to 2. And let dT be the corresponding rise in temperature of both
compartments. Then, using dQ nCdT
For the left compartment
dQ n1CdT
The process in the right compartment is isochoric
3R
dQ n 2Cv dT n 2 dT
2
3 n 2 3m 2
So, C R C R
2 n1 2m1
5
120. R , Diatomic
2
Let the weight placed on the piston W
1st step is adiabatic
i.e. PV C P CV
Gaseous State and Thermodynamics 93 Workbook – 3 | Solutions
For small changes, taking differential on both sides
dP CV 1 dV
W
Using dP P and dV A h1
A
W C
A h1
A V 1
W A h1 C
A V V
W A h1
(P ) . . . . (i)
A V
(1st + 2nd) step can be regarded as isothermal. So
PV = C dP CV 2dV
W C P
(1.4 h1) A 1.4 h1A . . . . (i)
A 2 V
V
From (i) and (ii) 1.4
fR 5R
i.e. gas is diatomic, Cv
2 2
121. (i) ln RT0 1 / 1 (ii) pV e 1 / pV = constant
(i) dQ nCdT
n T0
dQ dT Q n (nT )T
T 0
Q n n( ) . . . . (i)
nR nR
Also, U nCv T T ( 1) T0 . . . . (ii)
1 1
RT0 ( 1)
So, Q U W , and putting n = 1 W n
( 1)
(ii) Equation of process can be calculated by using the general formula of specific heat of an ideal gas
fR P dV
C
2 n dT
fR RT dV
Here
T 2 V dT
Solving, and converting to P, V gives
( 1)/ PV
PV e = const
122. Decreases 2.5 times
R R
Molar heat capcity for a polytropic process PV K const is given by C
1 K 1
7
But molar heat capacity is given C R and for a diatomic gas
5
1
Solving, we get K
3
For polytropic process
Gaseous State and Thermodynamics 94 Workbook – 3 | Solutions
V2
PV K C And it is given that 2
V
1
K K
P2 V1 1
2K
P1 V2 2
K 1
K 1 T1 1
Also TV C 2K 1 2 2
T2 T2
If n atoms strike a wall in time t, then change in momentum is given by:
p 2nm 0v x
p n
Average force on the wall due to the collisions: F 2m 0v x
t t
Rate of collision per unit area:
(n /t ) P
rC where P is gas pressure
A 2m 0v x
2
vrms P
Now, v x2 T rC
3 T
K 1 2
rC2 P2 T1 K
2 2 2 3 0.63
rC1 P1 T2
So rate of collision of molecules becomes 63% of its original value.
27 21 21 3
123. (i) PA PC P0 , PB P0 (ii) TA TB T0 , TC T0
8 4 4 2
17
(iii) 18P0 V0 (iv) WA P0 V0 , WB 0 (v) P0 V0
2
Since no exchange of heat is possible in part C, hence the process is adiabatic.
3/2
4V0 27
P1V1 P2V2 P0V03/ 2 PC PC P0
9 8
Pressure of part A and C must be equal for the piston to be in equilibrium.
27
PA PC P0
8
5V0 14V0
V A V0
9 9
Since no. of moles of gas in part A are constant
27 14V0
P0
P0V0 PAV A 8 9 T 21 T
A 0
T0 TA TA 4
21
TB T A T0 (piston I is conducting)
4
In part C
3
TCVC 1 T0V0 1 TC T0
2
Gaseous State and Thermodynamics 95 Workbook – 3 | Solutions
Change ion internal energy
21 17
U A n 2R T0 T0 P0V0
4 2
17
U B P0V0
2
3T0
U C n 2R T0 nRT0 P0V0
2
17
Heat flowing across piston 1 U B WC U B P0V0 .
2
Work done by gas in A change in internal energy of gas in C P0V0 .
Heat supplied by the gas U A U B WC
17 17
P0V0 P0V0 P0V0
2 2
18 P0V0
124. 2000N/m, 1295J
Kx
Pressure of gas, P P0
A
0.1
Work done by gas
PAdx
0
0.1
1 2
P0 A Kx dx P0 A 0.1 2 K 0.1
0
1
105 4 10 3 0.1 k 0.01 50
2
K
40 0.01 50 K 2000 N / m
2
Q U W
3
2 R 50 50 150 R 50 1295 J
2
Gaseous State and Thermodynamics 96 Workbook – 3 | Solutions
You might also like
- Gaskell Solution ManualDocument211 pagesGaskell Solution ManualJess Shih100% (1)
- Flat-Plate Solar Collectors For Water HeatingDocument271 pagesFlat-Plate Solar Collectors For Water HeatingelissiumNo ratings yet
- PVT Behavior of Fluida & EOSDocument53 pagesPVT Behavior of Fluida & EOSEka WahyuNo ratings yet
- Solutions: Solutions Manual For Introduction To The Thermodynamics of Materials 6Th Edition GaskellDocument228 pagesSolutions: Solutions Manual For Introduction To The Thermodynamics of Materials 6Th Edition Gaskellhamed pirboneh75% (4)
- Test Report PDFDocument60 pagesTest Report PDFMaria Marcano100% (2)
- LEED GA Exam Questions Set 6Document24 pagesLEED GA Exam Questions Set 6CEG BangladeshNo ratings yet
- Gaskell Laughlin SolutionsDocument233 pagesGaskell Laughlin Solutionsmojtaba pourbafrani100% (2)
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Turbomachinery Gopalakrishnan PDFDocument537 pagesTurbomachinery Gopalakrishnan PDFtpmuhammadtp93% (29)
- Sivacon Planning ManualDocument64 pagesSivacon Planning ManualFlo MircaNo ratings yet
- Dse720 Installation Instructions PDFDocument2 pagesDse720 Installation Instructions PDFanon_56872395776% (17)
- Gaseous State and Thermodynamics - Level 3 - DTS 10 - SolutionsDocument2 pagesGaseous State and Thermodynamics - Level 3 - DTS 10 - SolutionsSHIVAM KUMAR SINGHNo ratings yet
- Chapter 8 - Tut-3Document15 pagesChapter 8 - Tut-3Raghav ChhaparwalNo ratings yet
- Gaseous State and Thermodynamics - Level 3 - DTS 11 - SolutionsDocument3 pagesGaseous State and Thermodynamics - Level 3 - DTS 11 - SolutionsSHIVAM KUMAR SINGHNo ratings yet
- Turbomachinery: Summary EquationsDocument24 pagesTurbomachinery: Summary Equationsسرمد احمد علي ابراهيمNo ratings yet
- ALPS 2336 Physics Assignment SolutionsDocument12 pagesALPS 2336 Physics Assignment SolutionsSujal KapoorNo ratings yet
- HandtDocument3 pagesHandtvanuNo ratings yet
- ChE ThermodynamicsDocument49 pagesChE ThermodynamicsMiguel FelisildaNo ratings yet
- Facts and Formulae SheetDocument7 pagesFacts and Formulae SheethenryNo ratings yet
- THERMODYNAMICSDocument14 pagesTHERMODYNAMICSkamalesh949kamaleshNo ratings yet
- Chapt 4Document33 pagesChapt 4Ahmed AL-HarthiNo ratings yet
- Cap. 13Document284 pagesCap. 13Ent Qa NepalNo ratings yet
- Chemistry Final Step-C Solutions - Gaseous StateDocument6 pagesChemistry Final Step-C Solutions - Gaseous StateAnas KhalidNo ratings yet
- HW#3 Thermo PDFDocument19 pagesHW#3 Thermo PDFEdwinAndresRodriguezNo ratings yet
- Me ThermodynamicsDocument63 pagesMe ThermodynamicsGlenn Ray ErasmoNo ratings yet
- Jee Advanced Mock Test-13 Solution Paper-2 (14!04!2024)Document11 pagesJee Advanced Mock Test-13 Solution Paper-2 (14!04!2024)Tamanna SahuNo ratings yet
- FS Exam4Document2 pagesFS Exam4MasterSoupNo ratings yet
- CL 5002-Chemical Engineering Laboratory Ii Conduction: (A) Heat Transfer Through Composite Wall (B) Heat Transfer Though Lagged PipeDocument7 pagesCL 5002-Chemical Engineering Laboratory Ii Conduction: (A) Heat Transfer Through Composite Wall (B) Heat Transfer Though Lagged PipeAbhishek Raj ShekharNo ratings yet
- 2023 JEE Advanced Full Length 1 PAPER 2 Solutions Gen 3 To 7Document13 pages2023 JEE Advanced Full Length 1 PAPER 2 Solutions Gen 3 To 7raghvender147No ratings yet
- 1 Efek Panas ReviewDocument43 pages1 Efek Panas ReviewekkyNo ratings yet
- Exercise Ten Solutions - Heat Exchangers: Mechanical Engineering 375 Heat TransferDocument3 pagesExercise Ten Solutions - Heat Exchangers: Mechanical Engineering 375 Heat TransferhortalemosNo ratings yet
- Thermal Physics - II PDFDocument13 pagesThermal Physics - II PDFPriyanshu SharmaNo ratings yet
- Heat Source Systems: 1-Plane Wall With Heat SourcesDocument5 pagesHeat Source Systems: 1-Plane Wall With Heat SourcesAnonymous 2BJgxbxJNo ratings yet
- CondenserDocument5 pagesCondensersandeshNo ratings yet
- Lecture 37Document17 pagesLecture 37raynecoeNo ratings yet
- CondenserDocument1 pageCondenserhoangvubui4632No ratings yet
- Introduction To The Thermodynamics of Materials: Solutions Manual To AccompanyDocument20 pagesIntroduction To The Thermodynamics of Materials: Solutions Manual To AccompanyPrajjwal ChandNo ratings yet
- Full Download Physical Chemistry 2nd Edition Ball Solutions Manual PDF Full ChapterDocument36 pagesFull Download Physical Chemistry 2nd Edition Ball Solutions Manual PDF Full Chapterbristolodontosmac100% (17)
- Physics Advanced Level Problem Solving (ALPS-10) - SolutionDocument8 pagesPhysics Advanced Level Problem Solving (ALPS-10) - SolutionSwapnil MandalNo ratings yet
- EntropyDocument10 pagesEntropyShyam DevNo ratings yet
- Formula ListDocument3 pagesFormula ListRaihanNo ratings yet
- Lecture 2aDocument15 pagesLecture 2aKazem OsailyNo ratings yet
- Chapter 8 - Tut-4Document15 pagesChapter 8 - Tut-4Raghav ChhaparwalNo ratings yet
- Aditional Examples For Entropy ProductionDocument4 pagesAditional Examples For Entropy ProductionPaola AguileraNo ratings yet
- 01 State of Matter Gaseous StateDocument12 pages01 State of Matter Gaseous StateAkashGauravNo ratings yet
- K 800 K Kmole M KN 314 - 8 M KN 808 Kmole KG 01 - 28Document4 pagesK 800 K Kmole M KN 314 - 8 M KN 808 Kmole KG 01 - 28MH MerhiNo ratings yet
- Physical Chemistry 2nd Edition Ball Solutions ManualDocument36 pagesPhysical Chemistry 2nd Edition Ball Solutions Manualnaturedammitej0k0b100% (24)
- Lecture12 P2Document12 pagesLecture12 P2Phạm Thiên LongNo ratings yet
- Heat-03 - Objective & SubjectiveDocument19 pagesHeat-03 - Objective & SubjectiveRaju SinghNo ratings yet
- 19.43. I: Use: DentifyDocument4 pages19.43. I: Use: DentifyCoco LinNo ratings yet
- HW 06Document5 pagesHW 06Ya HooNo ratings yet
- Open Flow SystemDocument25 pagesOpen Flow Systempremium tresNo ratings yet
- R&AC Exp 01Document5 pagesR&AC Exp 01udhayarajNo ratings yet
- Thermo 1Document16 pagesThermo 1Arvin DalisayNo ratings yet
- HT 1Document2 pagesHT 1Gupta GurunadhGuptaNo ratings yet
- 13.12.2020 - JR Iit Co - Super Chaina - N120 - Adv - 2016 - P1 - Model Key PDFDocument11 pages13.12.2020 - JR Iit Co - Super Chaina - N120 - Adv - 2016 - P1 - Model Key PDFYugandher BadanaNo ratings yet
- Physical Chemistry 2nd Edition Ball Solutions ManualDocument17 pagesPhysical Chemistry 2nd Edition Ball Solutions Manualeirianarielo7a6rNo ratings yet
- ME 308 Machine Elements Ii: Journal Bearings - 2Document75 pagesME 308 Machine Elements Ii: Journal Bearings - 2xxxNo ratings yet
- Operation of 4-Stroke Cycle EngineDocument15 pagesOperation of 4-Stroke Cycle EngineNhật Thường Lê QuýNo ratings yet
- Full Physical Chemistry 2Nd Edition Ball Solutions Manual PDF Docx Full Chapter ChapterDocument36 pagesFull Physical Chemistry 2Nd Edition Ball Solutions Manual PDF Docx Full Chapter Chapterfijianrentage92q2x7100% (25)
- Dwnload Full Physical Chemistry 2nd Edition Ball Solutions Manual PDFDocument35 pagesDwnload Full Physical Chemistry 2nd Edition Ball Solutions Manual PDFsithprisus100% (11)
- CH 6-1Document19 pagesCH 6-1Aishahed mohamedNo ratings yet
- Dhw-Slide 2 Analisa HEDocument20 pagesDhw-Slide 2 Analisa HERavenSkullNo ratings yet
- Hints & Solutions: L Cos 2 2 Sin - Cos M 2 Cos - SinDocument9 pagesHints & Solutions: L Cos 2 2 Sin - Cos M 2 Cos - SinswarupNo ratings yet
- Ch4journbear2 200312773Document74 pagesCh4journbear2 200312773Mix TubeNo ratings yet
- Properties of Matter - Level - 3 - SolutionsDocument13 pagesProperties of Matter - Level - 3 - Solutionsdemolition squadNo ratings yet
- Wave Motion - Level 3Document8 pagesWave Motion - Level 3demolition squadNo ratings yet
- Introduction To Vector and Forces - Level 3Document3 pagesIntroduction To Vector and Forces - Level 3demolition squadNo ratings yet
- Liquids - Level 3 - SolutionsDocument12 pagesLiquids - Level 3 - Solutionsdemolition squadNo ratings yet
- Introduction To Vector and Force - Level 3 - SolutionDocument10 pagesIntroduction To Vector and Force - Level 3 - Solutiondemolition squadNo ratings yet
- Priority B Chapters PYQDocument146 pagesPriority B Chapters PYQdemolition squadNo ratings yet
- Lattice Vibrations: Monatomic Harmonic Chain Diatomic Harmonic Chain Thermal ConductivityDocument36 pagesLattice Vibrations: Monatomic Harmonic Chain Diatomic Harmonic Chain Thermal ConductivityliquefiedbutterNo ratings yet
- YST-SW315: Subwoofer SystemDocument23 pagesYST-SW315: Subwoofer Systemarmas1No ratings yet
- Atmscuw301 PDFDocument120 pagesAtmscuw301 PDFHeather CarterNo ratings yet
- Manual Burkert 1078-1 1078-2 PDFDocument35 pagesManual Burkert 1078-1 1078-2 PDFVũ PhongNo ratings yet
- Gamow-Teller Strength and Nuclear Deformation: MotivationDocument62 pagesGamow-Teller Strength and Nuclear Deformation: MotivationdwyphyNo ratings yet
- Standard Welding Procedures Specifications SWPS 2017-Nov-01Rev.0Document4 pagesStandard Welding Procedures Specifications SWPS 2017-Nov-01Rev.0Remmy Torres VegaNo ratings yet
- Physics GearsDocument101 pagesPhysics Gearssungjoo75No ratings yet
- M90 Users Manual 1Document77 pagesM90 Users Manual 1Jennifer EspartinezNo ratings yet
- Product Katalog CHJ 2023 CompressedDocument112 pagesProduct Katalog CHJ 2023 CompressedRusdi SalehNo ratings yet
- Sihp3wg4 Product ManualDocument2 pagesSihp3wg4 Product ManualPál HústalzNo ratings yet
- Optimizing Design & Control of Chilled Water Plants Part-2Document8 pagesOptimizing Design & Control of Chilled Water Plants Part-2GokhanUNLUNo ratings yet
- Equest Analysis of A Admin Block of An Institution, Pune: Guided By: Amey Naphade SirDocument14 pagesEquest Analysis of A Admin Block of An Institution, Pune: Guided By: Amey Naphade SirvaibhavNo ratings yet
- Comparison: Design PointDocument25 pagesComparison: Design PointDan PopescuNo ratings yet
- Lehe0886 00Document3 pagesLehe0886 00clavergara100% (1)
- Piping Installation Diagrams For Compact Steam Trapping StationsDocument2 pagesPiping Installation Diagrams For Compact Steam Trapping StationsRabindranath Hendy TagoreNo ratings yet
- Molecular Orbital TheoryDocument285 pagesMolecular Orbital Theorynadide100% (7)
- AVA2490ZXT: Performance Data SheetDocument10 pagesAVA2490ZXT: Performance Data SheetBazin TsegayeNo ratings yet
- BalancingValve Selection Guide PDFDocument1 pageBalancingValve Selection Guide PDFsahirprojectsNo ratings yet
- Index - 2017 - Integrated Gasification Combined Cycle IGCC Technologies PDFDocument23 pagesIndex - 2017 - Integrated Gasification Combined Cycle IGCC Technologies PDFrusdanadityaNo ratings yet
- Level 6Document71 pagesLevel 6hpoerbayaNo ratings yet
- Phy Chem 1 Lab Manualedited PDFDocument65 pagesPhy Chem 1 Lab Manualedited PDFJohn CaoileNo ratings yet
- Manuel en Englais de Moteur Pas A PasDocument82 pagesManuel en Englais de Moteur Pas A Pasminaud yann bileNo ratings yet