Professional Documents
Culture Documents
Vapour Power Cycles: Reheat Rankine Cycle
Uploaded by
NARAYANAN S0 ratings0% found this document useful (0 votes)
6 views13 pagesThe document discusses the reheated Rankine cycle. It describes the cycle process with steam being superheated and reheated at constant pressures before expanding. Calculations are shown for determining the enthalpy and entropy values at each state point. The efficiency of the cycle is calculated as 2.175 kg/kW hr based on the determined enthalpy values.
Original Description:
Original Title
18
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document discusses the reheated Rankine cycle. It describes the cycle process with steam being superheated and reheated at constant pressures before expanding. Calculations are shown for determining the enthalpy and entropy values at each state point. The efficiency of the cycle is calculated as 2.175 kg/kW hr based on the determined enthalpy values.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
6 views13 pagesVapour Power Cycles: Reheat Rankine Cycle
Uploaded by
NARAYANAN SThe document discusses the reheated Rankine cycle. It describes the cycle process with steam being superheated and reheated at constant pressures before expanding. Calculations are shown for determining the enthalpy and entropy values at each state point. The efficiency of the cycle is calculated as 2.175 kg/kW hr based on the determined enthalpy values.
Copyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 13
VAPOUR POWER
CYCLES
REHEAT RANKINE CYCLE
III SEM- ENGINEERING THERMODYNAMICS-UNIT IV VAPOUR POWER
1
CYCLEES/DR.R.SUDHAKARAN
III SEM- ENGINEERING THERMODYNAMICS-UNIT IV VAPOUR POWER
2
CYCLEES/DR.R.SUDHAKARAN
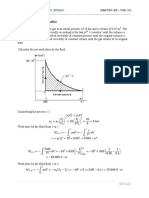
EFFICIENCY CALCULATION
III SEM- ENGINEERING THERMODYNAMICS-UNIT IV VAPOUR POWER
3
CYCLEES/DR.R.SUDHAKARAN
III SEM- ENGINEERING THERMODYNAMICS-UNIT IV VAPOUR POWER
4
CYCLEES/DR.R.SUDHAKARAN
III SEM- ENGINEERING THERMODYNAMICS-UNIT IV VAPOUR POWER
5
CYCLEES/DR.R.SUDHAKARAN
III SEM- ENGINEERING THERMODYNAMICS-UNIT IV VAPOUR POWER
6
CYCLEES/DR.R.SUDHAKARAN
At 150 bar and 5500C
Steam is superheated h1=3445.2 kJ/Kg s1=6.815 kJ/kg K=s2
40 bar
Sg2=6.069 kJ/kg K
4000C=6.773
5000C=7.091
1000C=0.318kJ/kg K
1kJ/kg K=(100/0.318)0C=314.460C
6.713 to 6.815 =6.815-6.713=0.102 kJ/kg K
For 0.102kJ/kg K =0.102*314.46 =32 0C
At 40 bar and s2=6.815 kJ/kg K T2=4320C
III SEM- ENGINEERING THERMODYNAMICS-UNIT IV VAPOUR POWER
7/18
CYCLEES/DR.R.SUDHAKARAN
AT 40 bar and 4320C h2
At 4000C=3215.7kJ/kg
At 5000C=3445.0kJ/kg
For 1000C increase of temp =enthalpy increases by 229.3kJ/kg
For 10C temp increase= enthalpy increases by 2.293 kJ/kg
For 320C temp increase = enthalpy increases by 73.37 kJ/kg
h2 At 40 bar & 4320C=3289.076kJ/kg
III SEM- ENGINEERING THERMODYNAMICS-UNIT IV VAPOUR POWER
8/18
CYCLEES/DR.R.SUDHAKARAN
Steam is reheated at constant pressure of 40 bar from 4320C to 5500C
Saturation temp at 40 bar = 250.30C
T3=5500C T3>Tsat
Steam is superheated at 3
From Steam Tables Enthalpy
III SEM- ENGINEERING THERMODYNAMICS-UNIT IV VAPOUR POWER
9/18
CYCLEES/DR.R.SUDHAKARAN
From Steam Tables Enthalpy at 40 bar
At 5000C =3445 kJ/kg
At 6000C=3672.8 kJ/kg
For 1000C increase of Temp=227.8kJ/kg increase of enthalpy
For 500C increase of Temp=113.9 kJ/kg increase of enthalpy
h3 at 40 bar and 5500C=3445+113.9=3558.9 kJ/kg
III SEM- ENGINEERING THERMODYNAMICS-UNIT IV VAPOUR POWER
10/18
CYCLEES/DR.R.SUDHAKARAN
From Steam Tables Entropy at 40 bar
At 5000C = 7.091 kJ/kg K
At 6000C=7.368 kJ/kg K
For 1000C increase of Temp=0.277 kJ/kg K increase of entropy
For 500C increase of Temp= 0.1385 kJ/kg K increase of entropy
s3 at 40 bar and 5500C=7.091+0.1385=7.2295 kJ/kg K=s4 at 0.1 bar
III SEM- ENGINEERING THERMODYNAMICS-UNIT IV VAPOUR POWER
11/18
CYCLEES/DR.R.SUDHAKARAN
At 0.1 bar sg4=8.151 kJ/kg K
s4<sg4
The steam is wet at 4
s4 =sf4 +x4sfg4
=0.877
h4=hf4+x4hfg4
h4=191.8+(0.877*2392.9)=2290.37 kJ/kg
III SEM- ENGINEERING THERMODYNAMICS-UNIT IV VAPOUR POWER
12/18
CYCLEES/DR.R.SUDHAKARAN
1424.654 kJ/kg
=2.175 kg/kW hr
III SEM- ENGINEERING THERMODYNAMICS-UNIT IV VAPOUR POWER
13/18
CYCLEES/DR.R.SUDHAKARAN
You might also like
- Mmchapter 7 Steam Generator and AuxilliariesDocument49 pagesMmchapter 7 Steam Generator and AuxilliariesKent Louie EyanaNo ratings yet
- Solution To Workshop 6-212 Power Cycles: School of Engineering and Sciences Thermodynamics Dr. Jorge Francisco EstelaDocument13 pagesSolution To Workshop 6-212 Power Cycles: School of Engineering and Sciences Thermodynamics Dr. Jorge Francisco EstelaChristian RengifoNo ratings yet
- Problems On Reheat CycleDocument8 pagesProblems On Reheat CycleMurvin VillarosaNo ratings yet
- Torrefiel Boiler, SecuredDocument61 pagesTorrefiel Boiler, SecuredKent Louie EyanaNo ratings yet
- Applied Thermodynamics Exam 2018 Wirh SolutionsDocument9 pagesApplied Thermodynamics Exam 2018 Wirh SolutionsFarouk BassaNo ratings yet
- Che198 Thermodynamics DrillsDocument8 pagesChe198 Thermodynamics DrillsTrebob GardayaNo ratings yet
- Power Cycles PDFDocument5 pagesPower Cycles PDFcarlverano0428No ratings yet
- Practical Task 4 ThermodynamicsDocument4 pagesPractical Task 4 ThermodynamicsSyfull musicNo ratings yet
- Aero Engineering Thermodynamics PDFDocument22 pagesAero Engineering Thermodynamics PDFSridharanNo ratings yet
- Vapour Power Cycles: Rankine CycleDocument22 pagesVapour Power Cycles: Rankine CycleNARAYANAN SNo ratings yet
- HOCM MAR Fez CoolerHeatBalanceDocument1 pageHOCM MAR Fez CoolerHeatBalanceOUSSAMA LAKHILI100% (2)
- Rohini 95807738964Document15 pagesRohini 95807738964aodmarahas56No ratings yet
- Me402 HW 4 Finals PDFDocument1 pageMe402 HW 4 Finals PDFMikko Omaña0% (2)
- PPEDocument1 pagePPEElla AlumbroNo ratings yet
- Thermo CycleDocument59 pagesThermo CycleDries CaersNo ratings yet
- Steam Generation Calculations of BoilerDocument5 pagesSteam Generation Calculations of BoilerRaza Un Nabi100% (1)
- Workshop 6-212 Power and Refrigeration CyclesDocument3 pagesWorkshop 6-212 Power and Refrigeration CyclesChristian RengifoNo ratings yet
- Powerplant and Industrial Plant Engineering Trial 1: 1 PointsDocument8 pagesPowerplant and Industrial Plant Engineering Trial 1: 1 PointsJerdNo ratings yet
- AEE 4 Basic Thermodynamics Final ExamDocument2 pagesAEE 4 Basic Thermodynamics Final ExamClint Jefferon C. ActubNo ratings yet
- Tinywow Screenshot 20240115 191943 45208809Document2 pagesTinywow Screenshot 20240115 191943 45208809Jlorenz AmandyNo ratings yet
- Ideal Rankine CycleDocument20 pagesIdeal Rankine CycleJasmin TulosaNo ratings yet
- Advanced Cycles JIPTDocument26 pagesAdvanced Cycles JIPTjp mishraNo ratings yet
- Applied ThermodynamicsDocument3 pagesApplied ThermodynamicsPRATAP SINGHNo ratings yet
- ME2202 ENGINEERING THERMODYNAMICS Nov-Dec 2012 Important Question V+ EditionDocument2 pagesME2202 ENGINEERING THERMODYNAMICS Nov-Dec 2012 Important Question V+ EditionPrasobh ShamohanNo ratings yet
- Emma MangarooDocument17 pagesEmma MangarooCharlotte BNo ratings yet
- 3-Vapour Compression SystemsDocument18 pages3-Vapour Compression SystemsUtkarsh SinghNo ratings yet
- Etd QB Set 2Document2 pagesEtd QB Set 2srinithims78No ratings yet
- Lab Report Specific HeatDocument5 pagesLab Report Specific HeatAhmad Shahir ShakriNo ratings yet
- Determination of Optimum Reheat Pressures For Single and Double Reheat Irreversible Rankine CycleDocument6 pagesDetermination of Optimum Reheat Pressures For Single and Double Reheat Irreversible Rankine CycleEdrielleNo ratings yet
- Lecture No.3 The Ideal Reheat Rankine CycleDocument12 pagesLecture No.3 The Ideal Reheat Rankine Cycledanielalejandro133No ratings yet
- Alexander Parkhomov Mar 26Document11 pagesAlexander Parkhomov Mar 26ecatworld100% (2)
- Chap 1 - Steam Power PlantDocument60 pagesChap 1 - Steam Power PlantMuhammad Qusyairi100% (2)
- Stirling Engine Efficiency (Experiment)Document11 pagesStirling Engine Efficiency (Experiment)Lukas BarauskasNo ratings yet
- Question Bank Thermal Engineering UPDATEDDocument6 pagesQuestion Bank Thermal Engineering UPDATEDIrfan ShaikhNo ratings yet
- c08 - Pending 8.36Document262 pagesc08 - Pending 8.36SeungMin LeeNo ratings yet
- Lampiran 2 Perhitungan Tugas Khusus 2Document7 pagesLampiran 2 Perhitungan Tugas Khusus 2Ainan AziziNo ratings yet
- HW 2Document13 pagesHW 2hat dogNo ratings yet
- Files MECH QB III ME6301 Engineering ThermodynamicsDocument15 pagesFiles MECH QB III ME6301 Engineering ThermodynamicsAnantha Kumar0% (1)
- Thermodynamics Problem Sheet 2Document4 pagesThermodynamics Problem Sheet 2Amna SaeedNo ratings yet
- MR2207 Atd1 Q&aDocument33 pagesMR2207 Atd1 Q&ajeffreysingh jdNo ratings yet
- Lab Report Specific Heat PDFDocument5 pagesLab Report Specific Heat PDFAhmad Shahir ShakriNo ratings yet
- SP Line III GenerationDocument5 pagesSP Line III Generationhmaza shakeelNo ratings yet
- Finding Cycle Efficiency, Work Ratio, Specific Steam Consumption, Rankine Efficiency, Relative Efficiency ThermodynamicsDocument3 pagesFinding Cycle Efficiency, Work Ratio, Specific Steam Consumption, Rankine Efficiency, Relative Efficiency ThermodynamicsJaswant Singh Sandhu63% (8)
- Measurement of Specific Heat For Various Agricultural ProductsDocument9 pagesMeasurement of Specific Heat For Various Agricultural ProductsSpetriani LamadauNo ratings yet
- Ian BAB 4Document6 pagesIan BAB 4NURUL SYUHADA BT ISMAIL HAJARNo ratings yet
- Assignment 4 - Jagonos, Ariel PDFDocument10 pagesAssignment 4 - Jagonos, Ariel PDFleno voiNo ratings yet
- Question Bank MechDocument102 pagesQuestion Bank MechKaradam PatelNo ratings yet
- Chem102fe 21920Document2 pagesChem102fe 21920Khiara Claudine EspinosaNo ratings yet
- VIP 5 Powerplant ExamDocument7 pagesVIP 5 Powerplant ExamJaybee LabraNo ratings yet
- Le4 LectureDocument1 pageLe4 LectureAsh KetchapNo ratings yet
- Dr. Malifeza Ass 01Document11 pagesDr. Malifeza Ass 01Fikirini MmaleNo ratings yet
- Thermo-2 Worked Examples and Worksheet On Vapor Power CycleDocument11 pagesThermo-2 Worked Examples and Worksheet On Vapor Power CycleÇãłl Mê MęlkãNo ratings yet
- ME1202-Tutorial 2Document1 pageME1202-Tutorial 2manarajNo ratings yet
- Problems On Regenerative CycleDocument6 pagesProblems On Regenerative CycleMurvin VillarosaNo ratings yet
- Steam Power Board Problems PDFDocument6 pagesSteam Power Board Problems PDFKim Niño FelisminoNo ratings yet
- Steam Power Board ProblemsDocument6 pagesSteam Power Board ProblemsCaguioa Mark Anthony G.100% (4)
- Analytical Chemistry of Zirconium and Hafnium: International Series of Monographs in Analytical ChemistryFrom EverandAnalytical Chemistry of Zirconium and Hafnium: International Series of Monographs in Analytical ChemistryNo ratings yet
- Heat Pumps: Solving Energy and Environmental ChallengesFrom EverandHeat Pumps: Solving Energy and Environmental ChallengesTakamoto SaitoNo ratings yet
- Engineering Thermodynamics Unit Iii Vapour Power CyclesDocument13 pagesEngineering Thermodynamics Unit Iii Vapour Power CyclesNARAYANAN SNo ratings yet
- Basic Mechanical Engineering Unit IV IC EnginesDocument16 pagesBasic Mechanical Engineering Unit IV IC EnginesNARAYANAN SNo ratings yet
- Properties of Pure Substances: Problems Using Steam TablesDocument13 pagesProperties of Pure Substances: Problems Using Steam TablesNARAYANAN SNo ratings yet
- Basic Mechanical Engineering Unit V Manufacturing ProcessDocument8 pagesBasic Mechanical Engineering Unit V Manufacturing ProcessNARAYANAN SNo ratings yet
- Vapour Power Cycles: Rankine CycleDocument22 pagesVapour Power Cycles: Rankine CycleNARAYANAN SNo ratings yet
- Basic Mechanical Engineering Unit IV IC EnginesDocument15 pagesBasic Mechanical Engineering Unit IV IC EnginesNARAYANAN SNo ratings yet
- Basic Concepts and First LawDocument11 pagesBasic Concepts and First LawNARAYANAN SNo ratings yet
- Basic Concepts and First LawDocument7 pagesBasic Concepts and First LawNARAYANAN SNo ratings yet
- Basic Concepts and First Law: Heat and Work TransferDocument8 pagesBasic Concepts and First Law: Heat and Work TransferNARAYANAN SNo ratings yet
- Basic Concepts and First LawDocument7 pagesBasic Concepts and First LawNARAYANAN SNo ratings yet
- Case Study Heat Transfer Enhancement by Nano FluidsDocument18 pagesCase Study Heat Transfer Enhancement by Nano FluidsNARAYANAN SNo ratings yet
- Case Study Micro Heat ExchangersDocument11 pagesCase Study Micro Heat ExchangersNARAYANAN SNo ratings yet
- Case Study - Biogas ProductionDocument18 pagesCase Study - Biogas ProductionNARAYANAN SNo ratings yet
- Oracle Database 11g - SQL Fundamentals I Vol3Document62 pagesOracle Database 11g - SQL Fundamentals I Vol3ralucap100% (1)
- ManualPX 80 Disc Stack Centrifuge PDFDocument78 pagesManualPX 80 Disc Stack Centrifuge PDFMuhammad UsamaNo ratings yet
- 18 Seam IvDocument509 pages18 Seam IvFroilan Espinosa80% (5)
- F2000 - Afm Sup11 Rev04 - 20100712Document36 pagesF2000 - Afm Sup11 Rev04 - 20100712rjohnson3773No ratings yet
- Eurton Electric Co., Inc.: AbbottDocument16 pagesEurton Electric Co., Inc.: Abbottjose navaNo ratings yet
- WITU2017 Show Update Vol1Document8 pagesWITU2017 Show Update Vol1quycoctuNo ratings yet
- First Prs EditedDocument38 pagesFirst Prs EditedMathew SebastianNo ratings yet
- Green EnergyDocument4 pagesGreen EnergyAlexandru AndreiNo ratings yet
- Colin Foote PHD Thesis March 2007Document326 pagesColin Foote PHD Thesis March 2007cfoote100% (3)
- Tie in Hot Work JHADocument4 pagesTie in Hot Work JHAmalik jahan100% (2)
- Cuponal Busbar Technical Data: AC/DC Current Ratings: ExtrusionsDocument4 pagesCuponal Busbar Technical Data: AC/DC Current Ratings: ExtrusionsPrakash19No ratings yet
- Fire & Explosion Risk Assessment (Kuliah Tamu Unair - 09 Juni 2012) BaruDocument50 pagesFire & Explosion Risk Assessment (Kuliah Tamu Unair - 09 Juni 2012) Baruveron_xiiiNo ratings yet
- Definitions: - Lists and Arrays - Nodes and Pointers - Single Linked Lists - Double Linked Lists - Circular ListsDocument41 pagesDefinitions: - Lists and Arrays - Nodes and Pointers - Single Linked Lists - Double Linked Lists - Circular ListsRuthwik H ParamNo ratings yet
- Proposal Report For CentrifugeDocument3 pagesProposal Report For CentrifugeDinesh KatochNo ratings yet
- G127 - Guidance For Reporting Uncertainty - Thunder Scientific - 10226-2Document3 pagesG127 - Guidance For Reporting Uncertainty - Thunder Scientific - 10226-2walterNo ratings yet
- Tank CleaningDocument24 pagesTank CleaningVipin Somasekharan100% (4)
- Nitrogen Cylinder 84L: SNS IG-100 Fire Suppression SystemDocument3 pagesNitrogen Cylinder 84L: SNS IG-100 Fire Suppression SystemNguyễn Minh ThiệuNo ratings yet
- Project Development of The Wayang Windu Unit 2 Geothermal Power PlantDocument6 pagesProject Development of The Wayang Windu Unit 2 Geothermal Power Plantbramono pramonoNo ratings yet
- 173 Altek 334A SpecDocument4 pages173 Altek 334A SpecroozbehxoxNo ratings yet
- Brochure UNIPVDocument20 pagesBrochure UNIPVPurushoth KumarNo ratings yet
- EightMedia Hammer - JsDocument10 pagesEightMedia Hammer - JsbinuramanNo ratings yet
- Basler DECSDocument12 pagesBasler DECSJulius Bandeleon BaylaNo ratings yet
- 17 Quality Characteristics EngineeringDocument20 pages17 Quality Characteristics EngineeringAries Sunanda100% (1)
- Operation & Maintenance Manual: Vertical Shaft Impact CrusherDocument56 pagesOperation & Maintenance Manual: Vertical Shaft Impact CrusherSergeyNo ratings yet
- Operational Manual: Pistol Fort - 28 Caliber 5.7x28 MMDocument8 pagesOperational Manual: Pistol Fort - 28 Caliber 5.7x28 MMluca ardenziNo ratings yet
- CH 3 Corrosion KineticsDocument50 pagesCH 3 Corrosion Kineticsميثة الغيثية100% (2)
- IGSS ManualDocument165 pagesIGSS Manualmenumorut12No ratings yet
- Voltage Sag and IndicesDocument142 pagesVoltage Sag and IndicesAnonymous MDkp0hnb3lNo ratings yet
- Yuanyang CableDocument3 pagesYuanyang CableAhmad SujaiNo ratings yet
- Case Analysis - 1Document1 pageCase Analysis - 1Bro HenryNo ratings yet