Professional Documents
Culture Documents
Introduction To Dyeing Fundamentals Novel Coloration and Sustainability
Uploaded by
Shahan Akhtar0 ratings0% found this document useful (0 votes)
10 views41 pagesOriginal Title
Lecture no. 7 and 8
Copyright
© © All Rights Reserved
Available Formats
PPTX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views41 pagesIntroduction To Dyeing Fundamentals Novel Coloration and Sustainability
Uploaded by
Shahan AkhtarCopyright:
© All Rights Reserved
Available Formats
Download as PPTX, PDF, TXT or read online from Scribd
You are on page 1of 41
Introduction to dyeing
Fundamentals Novel Coloration
and Sustainability
By Dr. M. Irfan Siyal
Lecture series for MS Textile Technology, NTU,
Faisalabad.
Contents
• Introduction to fundamentals and chemical
components of dyes
• Novel coloration technologies,
• IR dyeing,
• Supercritical dyeing,
• Effluent reduction
Introduction to Colorants
• A colorant is a substance capable of imparting colour to a
given substrate, such as cotton or any other textile
material, in which it is present
• Not all colorants are dyes
• A dye must be
– Soluble (or can be made soluble for their application),
– Substantive
– Absorbable (at fiber level)
• Pigments are colorants composed of particles that are
– Insoluble
– not substantive
– Pigment particles are large and do not penetrate into fiber
Attachment of dyes and pigments
to textiles
• When dyes are applied to fabrics, either by dyeing
or printing, they penetrate the fibres and are
attracted to them by primary forces (i.e. ionic or
covalent bonds) and secondary forces, such as
hydrogen bonds.
• When pigments are applied to textiles, mostly
through printing, they are mechanically bonded to
the fibre surface by resins called binders.
Characteristics of Dyes
• The four major characteristics of dyes are:
• intense colour
• solubility in water at some point during the
dyeing cycle
• some substantivity for the fibre being dyed
• reasonable fastness properties of the dyeing
produced.
Effect of physical structure of the fiber on dyeing
• Fiber molecules are grouped in the form of linear
polymeric chains.
• Generally these linear chains of polymers are
oriented along the axis of fibers or filaments.
• Dyeability of fibers depends on the orientation of
these molecular chains.
Effect of physical structure of
the fiber on dyeing
• In crystalline regions of
the fiber, the chains are
highly oriented posing
difficulties in the dye
penetration.
• In amorphous regions of
the fiber, the chains are
less oriented hence may
be considered as pores
allowing the dye
penetration.
Effect of physical structure of
the fiber on dyeing
• The size of pores in some fibers increase due to
fiber swelling in the presence of water, thus further
facilitating the dye penetration.
• Pore size of dry viscose fibers is 5 A (10−10 m) while
20-30* (10−10 m) when wet.
• Porosity of viscose rayon > mercerized cotton >
unmercerized cotton.
• In the same dyeing bath, colour intensity of viscose
rayon > mercerized cotton > unmercerized cotton.
Classification of dyes
For Cotton and other Cellulosics
• Direct Dyes
• Reactive Dyes
• Vat Dyes
• Sulfur Dyes
For Wool, Silk, Nylon and other Protein Based
• Acid Dyes
For Acrylic
• Basic Dyes
For Polyester
• Disperse Dyes
Basic Parts of a Dye Molecule
• Basic Parts- 1) Chromophores and 2) Auxochromes
– In chromophore, Chroma a greek word, means color and
phore is taken from pherein means to bear.
– Organic molecules containing on any one chromophore
becomes colored.
– In auxochromes, auxein means to increase.
– They increase intensity of color, solubility of dyes and
Colorfastness properties.
Chromophores
• Azo Group
• Triaryl Methane Group
• Nitroso Group
• Quinonoid Group
• Nitro Group
Auxochromes
• They intensify the shade depth of color.
• Additionally act as solubilizing agents b/c of polarity.
• Improve the color fastness (due to polarity).
• Hydroxyl group,
• Amino Group,
• Substituted Amino
• Sulfonic acid group, -SO3H, or
• Carboxylic acid group, -COOH, or more usually, the sodium salt
of these acids, -SO3-Na+ and -COO-Na+, respectively.
Examples
Introduction
• In textile processing coloration and functional
finishing are two necessary but traditionally
separated processes.
• Both processes repeatedly need wet treatments and
drying.
• They are therefore energy intensive and are
accompanied by effluents.
• Any attempt to combine both steps in one stage will
reduce energy as well as waste.
IR Dyeing
• The IR dyeing machine is a state of the art dyeing
instrument.
• This unit produces more accurate lab sample dyeing
with level and re-producible results and
accommodates up to 24 pots with a low liquor ratio
for diverse fibers.
• This unit moves the beakers in a circular rotation
with latest IR heating technology, to avoid uneven
heating for beakers.
IR Dyeing
• Temperature range 20-1400C
• ● Temperature gradient 0.5-
4.50C/min
• ● Temperature return speed 50C/min
• ● Liquor ratio 1:5
• ● Rotation speed 5-
70RPM(forth and reverse, two directions)
• ● Heating system Infra-
red
• ● Temperature control PLC
unit
• ● Cooling system
Forced air
• ● Max dyeing positions 24
Main advantages
• Eliminating glycerin contamination and cumbersome
beaker cleaning.
• Crease- and spot-free sample dyeings.
• Knitted and woven fabrics as well as yarn can be dyed.
• Cotton, wool and synthetic materials.
• Low liquor ratio: 1:5 fabric dyeing cotton.
• Stainless steel cabinet.
• Soda ash adding without opening the beakers.
• No glycerin or cooling water needed.
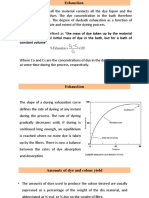
Diffusion
• Diffusion: movement of particles/molecules from an area of
high concentration to an area of low concentration.
• The material that diffuses could be a solid, liquid or gas.
• One of the main characteristics of diffusion is the movement
of molecules along the concentration gradient.
• Spraying of Perfume for fragrance is an example of
diffusion.
Effusion
• Effusion: movement of gas molecules through a
tiny hole,
• such as a hole in a balloon, into an evacuated space.
• Small puncture in tube of tyre etc.
• Effusion occurs or is facilitated by a difference of
pressures.
• Diffusion occurs due to difference in
concentrations.
What is supercritical fluid?
• Supercritical fluids have properties between those of a
gas and a liquid.
• A supercritical fluid can effuse through solids like a gas
and dissolve materials like a liquid.
• All supercritical fluids are completely miscible with each
other, so for a mixture a single phase can be guaranteed,
if the critical point of the mixture is exceeded.
• A supercritical fluid is any substance at a temperature
and pressure above its critical point, where distinct liquid
and gas phases do not exist.
Phase Diagram
Properties of supercritical fluids
• This can be rationalized by thinking that at high enough
temperatures (above the critical temperature) the
kinetic energy of the molecules is high enough to
overcome any intermolecular forces that would
condense the sample into the liquid phase.
• On the other hand, high enough pressures (above the
critical pressure) would not allow a sample to stay in
the pure gaseous state. Therefore, a balance between
these two tendencies is achieved and the substance
exists in a state between a gas and a liquid.
Critical temperature of fluids
• Gases can be converted to liquids by compressing the
gas at a suitable temperature.
• Gases become more difficult to liquefy as the
temperature increases because the kinetic energies of the
particles that make up the gas also increase.
• The critical temperature of a substance is the
temperature at and above which vapor of the substance
cannot be liquefied, no matter how much pressure is
applied.
• The critical pressure of a substance is the pressure
required to liquefy a gas at its critical temperature.
Critical temperature of fluids and
supercritical fluids
• To understand what happens at the critical point,
consider the effects of temperature and pressure on
the densities of liquids and gases, respectively.
• As the temperature of a liquid increases, its density
decreases.
• As the pressure of a gas increases, its density
increases.
• At the critical point, the liquid and gas phases have
exactly the same density, and only a single phase
exists. This single phase is called a supercritical fluid
Supercritical fluid textile dyeing technology
• Environmental compatibility of CO2
• There are many beneficial environmental effects when
supercritical carbon dioxide (scCO2) is applied as process
medium: CO2 does not contribute to smog, it has no
toxicity and the ozone layer is not damaged.
• It is also non-carcinogenic, non-flammable and non-toxic
(Jessop and Leitner, 1999); however, air with a CO2
content of more than 10% can be life-threatening if
breathed.
• The maximum allowable workplace concentration
(MAC) is 5000 ppm (Anon, 1992).
Supercritical fluid textile dyeing technology
• On the other hand, CO2 is known as a greenhouse gas and
there is an international growing concern about global
warming and its inter-relationship with levels of CO2 in the
air (Anon, 2003).
• Around 1800, before the industrial revolution, the CO2
concentration in the atmosphere was about 280 ppm and, in
1960, it went to 315 ppm.
• Since the mid-1900s, CO2 levels have been continually
increasing at an average annual rate of slightly more than 1
ppm, due to an increased combustion of fossil fuels and
natural processes.
• At present, the average CO2 concentration in the atmosphere
is about 380 ppm (Anon, 2003).
Supercritical fluid textile dyeing technology
• Processes which do not emit but apply CO2 as a
solvent have also been discussed very critically.
• Therefore, it is essential to investigate the sources of
CO2 and how it is recovered.
• Commercial quantities of CO2 are produced by
separating and purifying relatively CO2-rich gases
coming from combustion or biological processes
that would otherwise be released directly to the
atmosphere.
Supercritical fluid textile dyeing technology
• Common sources are hydrogen and ammonia plants,
magnesium production from dolomite, limekiln
operations and fermentation operations such as the
production of beer or the manufacture of ethanol
from corn (Anon, 2003).
• That means that processes such as supercritical fluid
dyeing do not increase CO2 emissions, but rather
provide an opportunity for recycling of waste CO2.
Supercritical fluid textile dyeing technology
• As the temperature and pressure rise along the
vapour–liquid coexistence line, liquid CO2 expands
and the two phases become less distinct forming a
so-called supercritical phase. Above the critical
point, the vapour–liquid line completely disappears.
Physicochemical properties of
CO2
• Pressure increase enhances solvent power and
solubility due to a higher density of the fluid.
• Viscosity of supercritical fluids is more gas-like
resulting in a reduced pressure loss (DP) due to
lower friction and transport limitations in technical
processes.
• The negligible surface tension leads to excellent
‘wetting’ properties.
Advantages and Limitations
• Because of the significance of PET and cotton, the
development of supercritical fluid dyeing
technologies worldwide is mainly focused on these
fibres and only to a minor extent on wool, silk,
polyamide and other technical fibres.
• While the dyeing of PET works very well in scCO2,
dyeing of polar fibres like cotton is still challenging
when high fastness properties and colour yields are
required.
Advantages and Limitations
• The limitations of dyeing natural fibres in scCO2 arise
from the inability of CO2 to break hydrogen bonds
(Kazarian et al., 1996;
• Saus et al., 1993d), the low degree of fiber swelling and
the low reactivity of the OH-bonds in cellulose in the
slightly acidic CO2 medium (Bach et al., 2002a).
• Furthermore, disperse dyes only show slight interactions
with polar fibres, leading to unacceptably low fastness
data, while reactive-, direct-, and acid dyes which are
used in conventional water dyeing are nearly insoluble in
scCO2.
Advantages and Limitations
• The most suitable scCO2 dyeing technology under
ecological aspects for natural fibres with all the
advantages known from PET dyeing is the
application of reactive disperse dyes.
• However, the dyes that have been applied in scCO2
dyeing experiments so far are not commercially
available yet and were custommade in the
laboratories of the different research groups.
Process steps for PET dyeing in scCO2
• The first step (Extraction I) represents the partial
extraction of spinning oils, followed by dyeing.
• Then extraction step II is started for removal of
adhering dye from the fabric surface and the inner of
the plant by rinsing with fresh cold scCO2.
• The temperature in the plant is decreased as fast as
possible below the glass transition temperature of the
polymer to avoid extraction of dye from the fibre bulk.
• Extracted dyes and spinning oils are precipitated in a
separator.
Process steps for PET dyeing in scCO2
Process steps for PET dyeing in scCO2
• At the end of the dyeing process, CO2 in the plant is
depressurized under liquefaction to the pressure in
the CO2 storage tank of about 50–55 bar.
• Remaining gaseous CO2 in the plant is released into
the atmosphere.
Non-aqueous Dyeing
• Dyeing from Air (Vapour-Phase Dyeing; Thermofixation)
• This continuous, pad–bake dyeing process, in which the
fibre is impregnated with disperse dyes, dried and then
baked for a short period of time at temperatures in the
region of 200 C, was introduced by Du Pont in 1949
under the trade name Thermosol.
• The process is based on the fact that very high rates of
dyeing can be achieved at elevated temperatures: for
example, a dyeing that may take several hours at 100 C
can be secured in <60 min at 130 C and in 20–30 s at 200
C.
Non-aqueous Dyeing
• Clearly shows the dramatic effect of increasing
temperature over the range 140–200 C on the colour
strength of C.I. Solvent Black 3 on PES fabric.
Non-aqueous Dyeing
• Although thermofixation can be used for the application of both vat
dyes and azoic colorants and to hydrophobic fibres other than PES,
utilisation of the process is mostly restricted to the application of
disperse dyes to PES and PES/cellulosic fibre blends.
• The concept of transferring disperse dyes via the vapour phase is
also employed in the transfer printing of PES and other
hydrophobic fibres, in which dye that is initially present on the
surface of an inert substrate (e.g. paper) is transferred at high
temperature (HT) to a dry fibre.
• This account concentrates on the thermofixation of disperse dyes
on PES fibres, the reader being directed to accounts of the
application of the process to PES/cellulosic fibre blends and
transfer printing.
References
• Physico-Chemical Aspects of Textile Coloration, Chapter 13.
• By: Stephen M. Burkinshaw
• Environmental aspects of textile dyeing, Chapter 5, Supercritical fluid
textile dyeing technology
• One-Bath Dyeing and Nonformaldehyde Durable Press Finishing of
Cotton Using Dialdehyde and a Monochlorotriazinyl Reactive Dye
• Hyung-Min Choi
• http://trrapid.com/ProductDetail/en-US/2057/ECO_DYER.aspx
• https://www.lab-pro.ch/laboratory/dyeing-machine-ird/
• http://www.km-textilemachines.com/ir-dyeing-machine.html#:~:text=The
%20IR%20dyeing%20machine%20is%20a%20state%20of%20the
%20art%20dyeing%20instrument.&text=This%20unit%20moves%20the
%20beakers,avoid%20uneven%20heating%20for%20beakers.
You might also like
- Disperse DyeDocument44 pagesDisperse DyeRatul HasanNo ratings yet
- Introduction To DyeingDocument74 pagesIntroduction To Dyeingsanjay shettiNo ratings yet
- Cellulosic dyeing methodsDocument19 pagesCellulosic dyeing methodsMuhammad Farooq KokabNo ratings yet
- Evolution of Dye TechnologyDocument28 pagesEvolution of Dye TechnologyNaeem MalikNo ratings yet
- Terminology and Dyeing Process FundamentalsDocument16 pagesTerminology and Dyeing Process Fundamentalsadali2020100% (2)
- Dyeing and FinishingDocument34 pagesDyeing and Finishingmuskan KhanNo ratings yet
- Dyeing and PrintingDocument94 pagesDyeing and Printingmahimabhat67% (3)
- Dyeing of Polyester With Disperse DyesDocument21 pagesDyeing of Polyester With Disperse Dyesirfanfakhar2No ratings yet
- Disperse Dyes ExplainedDocument3 pagesDisperse Dyes ExplainedMD saifu lislamNo ratings yet
- Disperse DyesDocument18 pagesDisperse DyesLiz AbyNo ratings yet
- Dyeing TheoryDocument11 pagesDyeing TheoryaliNo ratings yet
- dyeingDocument16 pagesdyeingsahidhasan0152No ratings yet
- Dyeing of PC Blended FabricDocument10 pagesDyeing of PC Blended FabricMian AnasNo ratings yet
- Wool DyeingDocument43 pagesWool Dyeingsandipsoni221811No ratings yet
- Direct Dyes: Submitted To: Dr. Bushra Nasar By: Saba ArshadDocument11 pagesDirect Dyes: Submitted To: Dr. Bushra Nasar By: Saba ArshadQuratul AinNo ratings yet
- Direct Dyes: Properties, Structure, Classification and Dyeing MechanismDocument11 pagesDirect Dyes: Properties, Structure, Classification and Dyeing MechanismQuratul AinNo ratings yet
- Chemical Testing DyeingDocument23 pagesChemical Testing DyeingMah GulNo ratings yet
- Dyeing Theory PDFDocument50 pagesDyeing Theory PDFRamnath Kumar Yadav100% (1)
- 3) Theory of DyeingDocument12 pages3) Theory of DyeingSanaullah MuradNo ratings yet
- Direct Dyes Lecture: Introduction, Properties, MechanismDocument27 pagesDirect Dyes Lecture: Introduction, Properties, MechanismrehanabbaciNo ratings yet
- Dyeing of TextilesDocument37 pagesDyeing of TextilesSenelisile Moyo100% (1)
- Dyeing of Polyester Fabric With Disperse DyesDocument4 pagesDyeing of Polyester Fabric With Disperse DyesKushagradhi Debnath100% (1)
- Intro To DyeingDocument26 pagesIntro To Dyeingchahat anejaNo ratings yet
- Textile Dyeing Introduction: Fiber Structure and Dyeing ProcessDocument10 pagesTextile Dyeing Introduction: Fiber Structure and Dyeing ProcessGanga DharanNo ratings yet
- Current Industrial Wastewater Treatment ProcessesDocument33 pagesCurrent Industrial Wastewater Treatment ProcessesSwateja MangleNo ratings yet
- National Textile University Faculty of Engineering & TechnologyDocument8 pagesNational Textile University Faculty of Engineering & TechnologyShahan AkhtarNo ratings yet
- IGCSE Chemistry Chapter 2 - Experimental TechniquesDocument27 pagesIGCSE Chemistry Chapter 2 - Experimental TechniquesVentus Tan75% (4)
- Retention ChemistryDocument86 pagesRetention Chemistryvivekbhuchem100% (1)
- Dyeing Machines and AdvancementDocument22 pagesDyeing Machines and AdvancementEngr Mujahid MehdiNo ratings yet
- Fundamentals of DyeingDocument138 pagesFundamentals of DyeingARYAN RATHORENo ratings yet
- Dyeing Mechanism ExplainedDocument21 pagesDyeing Mechanism ExplainedIftakharul IslamNo ratings yet
- Dyeing of Polyamide FibersDocument19 pagesDyeing of Polyamide FibersrajdewaanNo ratings yet
- Reactive DyesDocument24 pagesReactive DyesrehanabbaciNo ratings yet
- Water-Less Dyeing: A Sustainable ApproachDocument20 pagesWater-Less Dyeing: A Sustainable ApproachDhanashree Kudale100% (1)
- Physical Pharmacy: Gpat Online ClassesDocument82 pagesPhysical Pharmacy: Gpat Online Classesshripathy1-1No ratings yet
- Textile Testing MethodsDocument42 pagesTextile Testing MethodsNenadCirkovicNo ratings yet
- BASFDocument34 pagesBASFshashiNo ratings yet
- Dyes For CelluloseDocument18 pagesDyes For CelluloseAhmad ButtNo ratings yet
- Final Year PPT (Satwik)Document25 pagesFinal Year PPT (Satwik)Satwik Singh100% (1)
- Dyes MergedDocument304 pagesDyes Mergednadaelbeltagy4No ratings yet
- Introduction to Dyeing Textiles: Processes, Methods & TheoryDocument13 pagesIntroduction to Dyeing Textiles: Processes, Methods & TheoryImran100% (1)
- Fqa 3Document24 pagesFqa 3Nabarupa BoseNo ratings yet
- Dyeing of Cotton With Reactive DyesDocument39 pagesDyeing of Cotton With Reactive Dyestahir54No ratings yet
- Pore and Diffusion ModelDocument19 pagesPore and Diffusion ModelShumi NaharNo ratings yet
- 3-Characteristics of DyesDocument9 pages3-Characteristics of DyesWan M HilmiNo ratings yet
- Reactive Dye Mechanism and PropertiesDocument19 pagesReactive Dye Mechanism and Propertiesদীপ্তি হুমাইরাNo ratings yet
- Mercerizing Process ChangesDocument49 pagesMercerizing Process ChangesMatrix TeamNo ratings yet
- TD Assignment 2Document20 pagesTD Assignment 2palakNo ratings yet
- MercerisationDocument50 pagesMercerisationnikitaNo ratings yet
- Cationization of FabricDocument7 pagesCationization of Fabricraheem umer100% (1)
- Air Pollution Control Systems "Dust in The Wind": Omer Roberts Environmental EngineerDocument42 pagesAir Pollution Control Systems "Dust in The Wind": Omer Roberts Environmental EngineerYousef AlipourNo ratings yet
- Textile Chemical Processing RouteDocument74 pagesTextile Chemical Processing RouteOliyad EbbaNo ratings yet
- Fibre Structure and PropertiesDocument30 pagesFibre Structure and PropertiesAsifMK100% (1)
- Techniques in BiochemistryDocument64 pagesTechniques in BiochemistryShadowStormNo ratings yet
- Unit 3: Fabric Dyeing Process: 1.1. Vocabulary (T V NG)Document9 pagesUnit 3: Fabric Dyeing Process: 1.1. Vocabulary (T V NG)Linh NguyenNo ratings yet
- Technical Terms of Textile Dyeing: Muhammad Awais ImranDocument19 pagesTechnical Terms of Textile Dyeing: Muhammad Awais ImrantesfayergsNo ratings yet
- Acrylic Yarn DyeingDocument4 pagesAcrylic Yarn Dyeingsusheel deora0% (1)
- 11.3 Dyeing of Cotton With Reactive DyesDocument14 pages11.3 Dyeing of Cotton With Reactive DyesPraveen NagarajanNo ratings yet
- Fabric Dyeing MachineDocument8 pagesFabric Dyeing MachineSI ShakilNo ratings yet
- Advancements in Pretreatment of Textiles Using Enzymatic ProcessesDocument63 pagesAdvancements in Pretreatment of Textiles Using Enzymatic ProcessesShahan AkhtarNo ratings yet
- M9-10 Tapestry and Inkle WeavingDocument19 pagesM9-10 Tapestry and Inkle WeavingShahan AkhtarNo ratings yet
- Leno and Seersucker Fabric Weaving TechniquesDocument33 pagesLeno and Seersucker Fabric Weaving TechniquesZuhaib Ahmad0% (1)
- Textile Printing and AdvancementsDocument8 pagesTextile Printing and AdvancementsShahan AkhtarNo ratings yet
- Regulations Relating To The Use of Textile Dyes and ChemicalsDocument5 pagesRegulations Relating To The Use of Textile Dyes and ChemicalsShahan Akhtar100% (1)
- 3D Fabrics-02Document50 pages3D Fabrics-02Zuhaib AhmadNo ratings yet
- M10-11 Non Crimp Fabrics & Metal Mesh WeavingDocument25 pagesM10-11 Non Crimp Fabrics & Metal Mesh WeavingShahan AkhtarNo ratings yet
- Weaving Fundamentals: A Guide to Weave Structures and Fabric FormationDocument7 pagesWeaving Fundamentals: A Guide to Weave Structures and Fabric FormationShahan AkhtarNo ratings yet
- M4 - 3D Fabrics-02Document51 pagesM4 - 3D Fabrics-02Shahan AkhtarNo ratings yet
- Basic weaves and their propertiesDocument44 pagesBasic weaves and their propertiesShahan AkhtarNo ratings yet
- Spacer Fabrics: Dr. Zuhaib Ahmad Department of Materials and Testing National Textile UniversityDocument34 pagesSpacer Fabrics: Dr. Zuhaib Ahmad Department of Materials and Testing National Textile UniversityShahan AkhtarNo ratings yet
- M3 - 3D Fabrics-01 Lec-3Document36 pagesM3 - 3D Fabrics-01 Lec-3Shahan AkhtarNo ratings yet
- M6a Terry Towel WeavingDocument28 pagesM6a Terry Towel WeavingShahan AkhtarNo ratings yet
- Loom Motions ExplainedDocument8 pagesLoom Motions ExplainedShahan AkhtarNo ratings yet
- Lab 4Document4 pagesLab 4Shahan AkhtarNo ratings yet
- Module 6 (Looming) : Dr. Zuhaib AhmadDocument31 pagesModule 6 (Looming) : Dr. Zuhaib AhmadShahan AkhtarNo ratings yet
- Lab 4Document4 pagesLab 4Shahan AkhtarNo ratings yet
- L08: High Performance Polyamide FibersDocument20 pagesL08: High Performance Polyamide FibersShahan AkhtarNo ratings yet
- Dairy Farm 25 Cows Rs. 19.46 Million Jun-2017 PDFDocument29 pagesDairy Farm 25 Cows Rs. 19.46 Million Jun-2017 PDFRana Zeeshan UmerNo ratings yet
- Lab 4Document4 pagesLab 4Shahan AkhtarNo ratings yet
- L08: High Performance Polyamide FibersDocument20 pagesL08: High Performance Polyamide FibersShahan AkhtarNo ratings yet
- National Textile University Faculty of Engineering & Technology Advance Clothing Fall 2020Document5 pagesNational Textile University Faculty of Engineering & Technology Advance Clothing Fall 2020Shahan AkhtarNo ratings yet
- 2nd Lab...Document2 pages2nd Lab...Shahan AkhtarNo ratings yet
- TT-5063 Textile Lab ManualDocument2 pagesTT-5063 Textile Lab ManualShahan AkhtarNo ratings yet
- National Textile University Faculty of Engineering & TechnologyDocument8 pagesNational Textile University Faculty of Engineering & TechnologyShahan AkhtarNo ratings yet
- Recent Advances in SpinningDocument13 pagesRecent Advances in SpinningKali MuthuNo ratings yet
- Punjab livestock project improves rural incomesDocument33 pagesPunjab livestock project improves rural incomesFaizan ShahNo ratings yet
- M15 Innovations in KnittingDocument29 pagesM15 Innovations in KnittingShahan AkhtarNo ratings yet
- ElectrospinningDocument33 pagesElectrospinningShahan AkhtarNo ratings yet
- DearatorDocument3 pagesDearatorEDUARDONo ratings yet
- Stirring & Mixing: Types, Flow Patterns, Impeller SelectionDocument18 pagesStirring & Mixing: Types, Flow Patterns, Impeller SelectionHANNER ENRIQUE CANTILLO RUIZNo ratings yet
- Presentation ConocoPhillips Gas SeparatorDocument24 pagesPresentation ConocoPhillips Gas SeparatorEnyerberht Castañeda BritoNo ratings yet
- 22 - Gas Filters Separator - Series FS69Document6 pages22 - Gas Filters Separator - Series FS69Aalap DerasaryNo ratings yet
- Periodic Trends and Properties of ElementsDocument29 pagesPeriodic Trends and Properties of ElementsRamya. R100% (1)
- Energy Balance On W1Document3 pagesEnergy Balance On W1Kricel MaqueraNo ratings yet
- STATOIL-Slug ControlDocument28 pagesSTATOIL-Slug Controlviswalng100% (1)
- Penetration Tests According To The ISO 11140Document41 pagesPenetration Tests According To The ISO 11140masthan6yNo ratings yet
- Floquat™ FL 3150 - TDS - MSDocument1 pageFloquat™ FL 3150 - TDS - MSJavier Arancibia MartinezNo ratings yet
- Statistical PhysicsDocument21 pagesStatistical PhysicsSaswata RoyNo ratings yet
- AIGA 016 - 05 Safety Features of Portable Cryo Liq ContainersDocument6 pagesAIGA 016 - 05 Safety Features of Portable Cryo Liq ContainersMoisés Camarillo HaroNo ratings yet
- Topics 10-2 Relationship Between Pressure and DensityDocument4 pagesTopics 10-2 Relationship Between Pressure and DensityHinata CosaNo ratings yet
- API SealDocument13 pagesAPI SealjasminneeNo ratings yet
- Mechanics of FluidsDocument64 pagesMechanics of FluidsShiva U100% (2)
- Stripper DesignDocument8 pagesStripper Designmohamed0% (1)
- PHYSICAL CHEMISTRY-phase Diagram 3 ComponentsDocument22 pagesPHYSICAL CHEMISTRY-phase Diagram 3 ComponentsMuhammad YanuarNo ratings yet
- Figure 1. Simple Distillation Set-UpDocument2 pagesFigure 1. Simple Distillation Set-UpJacques TuckerNo ratings yet
- Batangas State University: Republic of The PhilippinesDocument5 pagesBatangas State University: Republic of The PhilippinesMelvin Pogi138No ratings yet
- Example 1Document8 pagesExample 1jgolloberNo ratings yet
- XLL - Project - AdsorptionDocument12 pagesXLL - Project - AdsorptionxxxNo ratings yet
- Achiever DLP Unit Test 1 SoDocument11 pagesAchiever DLP Unit Test 1 SoJane ParkNo ratings yet
- Solucion Del Libro JacbsonDocument54 pagesSolucion Del Libro JacbsonXavi Di CardiNo ratings yet
- 4 Earth's Subsystem PDFDocument31 pages4 Earth's Subsystem PDFAyesha YusopNo ratings yet
- Ammonia Plant Design For 1 MtpaDocument43 pagesAmmonia Plant Design For 1 MtpaPrateek Mall67% (3)
- Impact of Source To Drain Tunneling On The Ballistic Performance of Si Ge GaSb and GeSn Nanowire p-MOSFETsDocument8 pagesImpact of Source To Drain Tunneling On The Ballistic Performance of Si Ge GaSb and GeSn Nanowire p-MOSFETsYadav YNo ratings yet
- Assignment-3 I SEM IT-B 2023-24Document1 pageAssignment-3 I SEM IT-B 2023-24varunkumargongallaNo ratings yet
- Physics Neet PYQs Class 11 Chapter 13Document4 pagesPhysics Neet PYQs Class 11 Chapter 13LONE WOLFNo ratings yet
- ME010 704: Refrigeration and Air ConditioningDocument2 pagesME010 704: Refrigeration and Air ConditioningAnonymous VDnLHNG7QQNo ratings yet
- Flow CorrelationsDocument24 pagesFlow CorrelationsAliNo ratings yet
- Process Air ChillersDocument24 pagesProcess Air Chillersciccio100% (1)