Professional Documents
Culture Documents
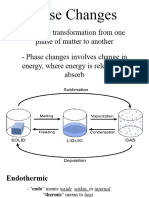
Heat
Uploaded by
M KarimCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Heat
Uploaded by
M KarimCopyright:
Available Formats
Conceptual Physics
11th Edition
Chapter 21:
TEMPERATURE, HEAT AND EXPANSION
© 2010 Pearson Education, Inc.
After this lecture, you should be able to Explain:
Temperature
Heat
Specific Heat Capacity
Thermal Expansion
© 2010 Pearson Education, Inc.
Temperature
A number that corresponds to the warmth or coldness of an object
Measured by a thermometer
Is a per-particle property
No upper limit
Definite limit on lower end
© 2010 Pearson Education, Inc.
Temperature
Temperature is proportional to the average translational
kinetic energy per particle in a substance.
Gas—how fast the gas particles are bouncing
Liquid—how fast particles slide and jiggle past one another
Solid—how fast particles move as they vibrate and jiggle in
place
© 2010 Pearson Education, Inc.
Temperature
Thermometer
Measures temperature by expansion or contraction of a
liquid (mercury or colored alcohol)
Reading occurs when the thermometer and the object
reach thermal equilibrium (having the same average
kinetic energy per particle)
Infrared thermometers operate by sensing IR radiation
© 2010 Pearson Education, Inc.
Temperature scale
Celsius scale named after Anders Celsius (1701
–1744).
0C for freezing point of water to 100C for boiling
point of water
Fahrenheit scale named after G. D. Fahrenheit
(1686–1736).
32F for freezing point of water to 212F for boiling point
of water
Kelvin scale named after Lord Kelvin (1824–1907).
273 K for freezing point of water to 373 K for boiling
point of water
0 K (-273 C) at absolute zero; same size degrees as
Celsius scale
kelvins, rather than degrees, are used
© 2010 Pearson Education, Inc.
Temperature
To say that body A has a higher temperature than body B is to
say that body A has more
A. internal energy.
B. mass.
C. kinetic energy per particle.
D. potential energy.
© 2010 Pearson Education, Inc.
Temperature
To say that body A has a higher temperature than body B
is to say that body A has more
A. internal energy.
B. mass.
C. kinetic energy per particle.
D. potential energy.
© 2010 Pearson Education, Inc.
Heat
Internal energy transferred from one thing to another due to a
temperature difference
Internal energy in transit
Flow of internal energy
From a high-temperature substance to a low-temperature
substance until thermal equilibrium is reached
Internal energy never flows unassisted from a low-temperature
to a high-temperature substance
© 2010 Pearson Education, Inc.
Heat
If a red-hot thumbtack is immersed in warm water, the
direction of heat flow will be from the
A. warm water to the red-hot thumbtack.
B. red-hot thumbtack to the warm water.
C. There will be no heat flow.
D. Not enough information.
© 2010 Pearson Education, Inc.
Heat
If a red-hot thumbtack is immersed in warm water, the
direction of heat flow will be from the
A. warm water to the red-hot thumbtack.
B. red-hot thumbtack to the warm water.
C. There will be no heat flow.
D. Not enough information.
© 2010 Pearson Education, Inc.
Quantity of Heat
Quantity of heat
Measured in joules or calories
4.18 joules of heat are required to change the
temperature of 1 gram of water by 1 Celsius degree
4.18 joules = 1 calorie
© 2010 Pearson Education, Inc.
Quantity of Heat
Energy ratings of foods and fuels are determined from energy
released when they are burned.
Unit of energy, the Calorie, is common for foods.
Heat unit for labeling food
kilocalorie or 1000 calories called a
Calorie
heat needed to change the temperature
of 1 kg of water by 1C
© 2010 Pearson Education, Inc.
Quantity of Heat
The same quantity of heat is added to different amounts of
water in two equal-size containers. The temperature of the
smaller amount of water
A. decreases more.
B. increases more.
C. does not change.
D. Not enough information.
© 2010 Pearson Education, Inc.
Quantity of Heat
The same quantity of heat is added to different amounts of water in
two equal-size containers. The temperature of the smaller amount
of water
A. decreases more.
B. increases more.
C. does not change.
D. Not enough information.
© 2010 Pearson Education, Inc.
Quantity of Heat
You heat a half-cup of tea and its temperature rises by 4C. How
much will the temperature rise if you add the same amount of
heat to a full cup of tea?
A. 0C
B. 2C
C. 4C
D. 8C
© 2010 Pearson Education, Inc.
Quantity of Heat
You heat a half-cup of tea and its temperature rises by 4C. How
much will the temperature rise if you add the same amount of
heat to a full cup of tea?
A. 0C
B. 2C
C. 4C
D. 8C
© 2010 Pearson Education, Inc.
Specific Heat Capacity
Defined as the quantity of heat required to change the
temperature of a unit mass of the substance by 1
degree Celsius
Like thermal inertia—resistance of a substance to a
change in temperature
© 2010 Pearson Education, Inc.
Specific Heat Capacity
Different substances have different thermal capacities for
storing energy.
Example:
• Takes about 2 minutes to raise the temperature of an
iron pot of water to boiling temperature
• Takes less than 1 minute to raise the temperature of
the same quantity of water in a silver pot to boiling
temperature
© 2010 Pearson Education, Inc.
Specific Heat Capacity
Equal masses of different materials required different quantities of
heat to change their temperatures by a specified number of degrees.
1 gram of water requires 1 calorie of energy to raise the temperature
1 degree Celsius.
1 gram of iron requires 1/8 as much energy for the same temperature
increase. Therefore, water absorbs more heat than iron for the same
change in temperature. Water has a higher specific heat.
© 2010 Pearson Education, Inc.
Specific Heat Capacity
The high specific heat capacity of water
Has higher capacity for storing energy than almost any other
substance.
Involves various ways that energy can be absorbed.
- Increases the jiggling motion of molecules, which raises
the temperature.
- Increases the amount of internal vibration or rotation
within the molecules, which becomes potential energy and
doesn’t raise temperature
- Water molecules can absorb energy without increasing
translational kinetic energy
© 2010 Pearson Education, Inc.
Specific Heat Capacity
Which has the higher specific heat capacity, water or land?
A. Water
B. Land
C. Both of the above are the same.
D. None of the above.
© 2010 Pearson Education, Inc.
Specific Heat Capacity
Which has the higher specific heat capacity, water or land?
A. Water
B. Land
C. Both of the above are the same.
D. None of the above.
*A substance with small temperature changes for large
heat changes has a high specific heat capacity. Water takes
much longer to heat up in the sunshine than does land. This
difference is a major influence on climate.
© 2010 Pearson Education, Inc.
You might also like
- Thermochemistry: Purpose of The ExperimentDocument20 pagesThermochemistry: Purpose of The ExperimentHassan Ali0% (1)
- Heat NotesDocument105 pagesHeat NotesNuan Ting NgNo ratings yet
- Into The Unknown 21 Doc PDFDocument9 pagesInto The Unknown 21 Doc PDFFernando AlbuquerqueNo ratings yet
- Specific Heat Capacity and Specific Latent Heat PDFDocument3 pagesSpecific Heat Capacity and Specific Latent Heat PDFNyak PereraNo ratings yet
- Chapter 4 Heat Teacher's GuideDocument34 pagesChapter 4 Heat Teacher's GuidehermanwongNo ratings yet
- Science: Modified Strategic Intervention MaterialDocument32 pagesScience: Modified Strategic Intervention MaterialMar Angelo TangcangcoNo ratings yet
- Brock Planetary Declination SDocument6 pagesBrock Planetary Declination SDositheus Seth100% (2)
- 4.1 Heat: Thermal EquilibriumDocument6 pages4.1 Heat: Thermal Equilibriumfizikmozac0% (1)
- WLP-Week 6Document15 pagesWLP-Week 6Justin Abad Fernandez100% (1)
- Science 10 Quarter 2 Module 4Document6 pagesScience 10 Quarter 2 Module 4Jess Anthony Efondo100% (4)
- Conceptual Physics: 11 EditionDocument41 pagesConceptual Physics: 11 Editionthamer hamadNo ratings yet
- Heat and Temperature PDFDocument94 pagesHeat and Temperature PDF• Nate •0% (1)
- 10 Class Physics Material: Reflection of Light by Different SurfacesDocument1 page10 Class Physics Material: Reflection of Light by Different SurfacesramprasadNo ratings yet
- Larry D. Buban, PH.D Science Education - Physics Cas - WvsuDocument49 pagesLarry D. Buban, PH.D Science Education - Physics Cas - WvsuHannah Grace Romano ViceralNo ratings yet
- Faster. Rate of Energy Transfer: 4.1: Understanding Thermal EquilibriumDocument6 pagesFaster. Rate of Energy Transfer: 4.1: Understanding Thermal EquilibriumZalini AbdullahNo ratings yet
- ICSE X Physics - Chap 12 (Calorimetry)Document10 pagesICSE X Physics - Chap 12 (Calorimetry)mohammedumar7864521No ratings yet
- TUP Application Demo (Quantity of Heat)Document28 pagesTUP Application Demo (Quantity of Heat)Coleen AmadoNo ratings yet
- HEAT p3p4 StudentsDocument23 pagesHEAT p3p4 StudentsSharvinder SinghNo ratings yet
- Bab 3 Heat (ICO7)Document4 pagesBab 3 Heat (ICO7)anung edy nugrohoNo ratings yet
- Activity 5Document5 pagesActivity 5Yani CenaNo ratings yet
- 8 Nibqis PKEZpp FDPWDUqDocument24 pages8 Nibqis PKEZpp FDPWDUqmrockzedzNo ratings yet
- 11 Phy w12 NotesDocument9 pages11 Phy w12 NotesPhilip FanaiNo ratings yet
- Heat (Add Science) OkDocument35 pagesHeat (Add Science) OkJaswardi Anwar Bin Md Yaacob� IPGKKBNo ratings yet
- Handout Temperature and Heat 1Document10 pagesHandout Temperature and Heat 1NinaRicaR.RamosNo ratings yet
- Calorimetry SynopsisDocument4 pagesCalorimetry Synopsissreevaishnava01No ratings yet
- P3 Matter Revision Questions 2 8 9Document17 pagesP3 Matter Revision Questions 2 8 9zubmalik09No ratings yet
- Physics Heat-EnglishDocument2 pagesPhysics Heat-EnglishGoogle AccountNo ratings yet
- Diaz - SC33 - Activity 6 B - SpecificHeatCapacityDocument10 pagesDiaz - SC33 - Activity 6 B - SpecificHeatCapacityJohn Rafael DiazNo ratings yet
- Lighting Dan HeatDocument118 pagesLighting Dan HeatAndri Fayrina RamadhaniNo ratings yet
- The Sound of Laughter Is Like The Vaulted Dome of A Temple of HappinessDocument2 pagesThe Sound of Laughter Is Like The Vaulted Dome of A Temple of HappinessRanulfo MayolNo ratings yet
- Chapter 11 - Calorimetry - Selina Solutions Concise Physics Class 10 ICSE - KnowledgeBoatDocument70 pagesChapter 11 - Calorimetry - Selina Solutions Concise Physics Class 10 ICSE - KnowledgeBoatskjNo ratings yet
- Lesson 3 New - X STDDocument6 pagesLesson 3 New - X STDkratosthegodkiller51No ratings yet
- Thermal 1 Heat Vs TemperatureDocument16 pagesThermal 1 Heat Vs TemperatureBrian TotmanNo ratings yet
- P 9 T2 06 HeatDocument17 pagesP 9 T2 06 HeatParth KhillareNo ratings yet
- 4.2 Specific Heat Cap 2020Document51 pages4.2 Specific Heat Cap 2020zaim aqeelNo ratings yet
- Physics Form 4 Chapter 4 Heat NoteDocument4 pagesPhysics Form 4 Chapter 4 Heat Notecyric wongNo ratings yet
- HeatDocument6 pagesHeatsrik1376No ratings yet
- Unit 5 ThermodynamicsDocument6 pagesUnit 5 Thermodynamicsමේනුක සූවින්දNo ratings yet
- Specific Heat CapacityDocument14 pagesSpecific Heat CapacityAidan KNo ratings yet
- Chapter 2 IGCSE - ActualDocument7 pagesChapter 2 IGCSE - ActualNajia UmarNo ratings yet
- CaliometryDocument14 pagesCaliometryshivanshkumarsahu07No ratings yet
- Thermal PhysicsDocument13 pagesThermal PhysicsKhawaja EshaNo ratings yet
- Als Pass 34: Roficiency Ssessment Upplementary HeetDocument1 pageAls Pass 34: Roficiency Ssessment Upplementary HeetALS-MP CARNo ratings yet
- Thermochemistry: Purpose of The ExperimentDocument20 pagesThermochemistry: Purpose of The ExperimentAngel LacsonNo ratings yet
- Heat-I: Theory and Exercise BookletDocument39 pagesHeat-I: Theory and Exercise BookletMD CHHIMPANo ratings yet
- HURDCO International SchoolDocument3 pagesHURDCO International SchoolWakif Khan PrantoNo ratings yet
- Haba Dan TenagaDocument20 pagesHaba Dan TenagahisharuddinNo ratings yet
- Science 8 Q1 W6Document15 pagesScience 8 Q1 W6Joanabel DechosaNo ratings yet
- Topic3 - SL&HL - MC (4 Files Merged)Document52 pagesTopic3 - SL&HL - MC (4 Files Merged)cynwuyxNo ratings yet
- HeatDocument163 pagesHeatSrMoonSengChoo100% (1)
- 6i Ag U2ptDocument3 pages6i Ag U2ptadhrit.mahatoNo ratings yet
- G-8 Physics Unit 4 Note - 07-08-13796168803685 - Gobe - 3Document9 pagesG-8 Physics Unit 4 Note - 07-08-13796168803685 - Gobe - 3AbebechNo ratings yet
- Physics ReviewDocument159 pagesPhysics ReviewSinned ArgalesNo ratings yet
- Lesson 6 Heat & TemperatureDocument18 pagesLesson 6 Heat & TemperatureRonnie AbsalonNo ratings yet
- General Chemistry 2 Q1 Lesson 5 Endothermic and Exotheric Reaction and Heating and Cooling CurveDocument19 pagesGeneral Chemistry 2 Q1 Lesson 5 Endothermic and Exotheric Reaction and Heating and Cooling CurveJolo Allexice R. PinedaNo ratings yet
- Thermal Energy and Heat What Is The Relationship Between Heat and Temperature?Document25 pagesThermal Energy and Heat What Is The Relationship Between Heat and Temperature?MinduliNo ratings yet
- Topic 3 and 10 Question Set 1Document14 pagesTopic 3 and 10 Question Set 1Michael lIuNo ratings yet
- Refrigeration 101: Rusty Walker, Corporate Trainer HillphoenixDocument56 pagesRefrigeration 101: Rusty Walker, Corporate Trainer HillphoenixDimson B. CabacangNo ratings yet
- Basic Chemistry: 3.5 Specific HeatDocument31 pagesBasic Chemistry: 3.5 Specific HeatCowo Rasa AlpukatNo ratings yet
- (1800) Lecture Notes 1 Calorimetery and Thermal Expansion eDocument21 pages(1800) Lecture Notes 1 Calorimetery and Thermal Expansion eSreyas KothaNo ratings yet
- UntitledDocument10 pagesUntitledaliNo ratings yet
- Israel StandardDocument15 pagesIsrael StandardDũng Bùi Đức100% (1)
- PDFDocument8 pagesPDFDocNo ratings yet
- Calculate Cable Size and Voltage Drop Electrical Notes Articles PDFDocument10 pagesCalculate Cable Size and Voltage Drop Electrical Notes Articles PDFRavi SharmaNo ratings yet
- Epilepsy Lecture NoteDocument15 pagesEpilepsy Lecture Notetamuno7100% (2)
- DHT, VGOHT - Catloading Diagram - Oct2005Document3 pagesDHT, VGOHT - Catloading Diagram - Oct2005Bikas SahaNo ratings yet
- Age ProblemDocument31 pagesAge ProblemKenny CantilaNo ratings yet
- Advanced Steel Structure Concepts: 2 MonthsDocument4 pagesAdvanced Steel Structure Concepts: 2 MonthsAnkit SoniNo ratings yet
- Manual Generador CAT C15 IbaguéDocument6 pagesManual Generador CAT C15 IbaguéAndres VargasNo ratings yet
- EY Enhanced Oil RecoveryDocument24 pagesEY Enhanced Oil RecoveryDario Pederiva100% (1)
- Smart Locker - A Sustainable Urban Last-Mile Delivery Solution: Benefits and Challenges in Implementing in VietnamDocument14 pagesSmart Locker - A Sustainable Urban Last-Mile Delivery Solution: Benefits and Challenges in Implementing in VietnamQuynh LeNo ratings yet
- Vol07 1 PDFDocument275 pagesVol07 1 PDFRurintana Nalendra WarnaNo ratings yet
- Iloilo City Regulation Ordinance 2006-010Document4 pagesIloilo City Regulation Ordinance 2006-010Iloilo City CouncilNo ratings yet
- Wjec Biology SpectificaionDocument93 pagesWjec Biology SpectificaionLucy EvrettNo ratings yet
- Amc 20-21Document33 pagesAmc 20-21Vasco M C SantosNo ratings yet
- Diels-Alder Reaction: MechanismDocument5 pagesDiels-Alder Reaction: MechanismJavier RamirezNo ratings yet
- Production Technology of Dragon FruitDocument6 pagesProduction Technology of Dragon FruitAbhinash MoirangthemNo ratings yet
- En LF Drivers 10nw76 8Document3 pagesEn LF Drivers 10nw76 8ChrisNo ratings yet
- Calabano Clinical Bacteriology Exercise 1Document5 pagesCalabano Clinical Bacteriology Exercise 1MarkJasperCalabanoNo ratings yet
- Ge Druck PTX 7535Document2 pagesGe Druck PTX 7535ICSSNo ratings yet
- ScheduleDocument1 pageScheduleparag7676No ratings yet
- 2.4 Assembly ManualDocument139 pages2.4 Assembly Manualgustavo dlsNo ratings yet
- PalmistryDocument116 pagesPalmistrymarinoyogaNo ratings yet
- 1 Name of Work:-Improvement of Epum Road (Northern Side) Connecting With Imphal-Saikul Road I/c Pucca DrainDocument1 page1 Name of Work:-Improvement of Epum Road (Northern Side) Connecting With Imphal-Saikul Road I/c Pucca DrainHemam PrasantaNo ratings yet
- Present Arlypon VPCDocument1 pagePresent Arlypon VPCErcan Ateş100% (1)
- PECI 405 ECPP 7th Sem CivilDocument96 pagesPECI 405 ECPP 7th Sem CivilYasaswi AkkirajuNo ratings yet
- Output Process Input: Conceptual FrameworkDocument4 pagesOutput Process Input: Conceptual FrameworkCHRISTINE DIZON SALVADORNo ratings yet
- NCERT Solutions For Class 12 Maths Chapter 10 Vector AlgebraDocument51 pagesNCERT Solutions For Class 12 Maths Chapter 10 Vector AlgebraKavin .J.S (KingK)No ratings yet