Professional Documents
Culture Documents
M Lote LP Lef 19 002 N D
Uploaded by
Brendapaez3Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
M Lote LP Lef 19 002 N D
Uploaded by
Brendapaez3Copyright:
Available Formats
Instrument:MP-15 Sequence:BETAHISTINA DICLORHIDRATO AGO-31-20 Page 1 of 6
LABORATORIO FARBROQUIM S.A.S
Injection Date/Time: 01/Sep/20 03:30
Processing Method: BETAHISTINA DICLORHIDRATO AGO-31-20
Instrument name: MP-15 Injection Type: Unknown
Injection Name: 01/Sep/2020 03:30 Injection Volume: 25.00
Comment: LEFLUX 8 mg TAB EN T3 TRILAMINADO
Directory: Instrument Data\MP-15\AGOSTO 2020
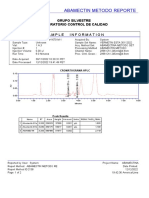
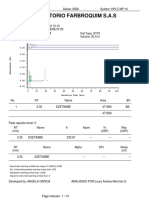
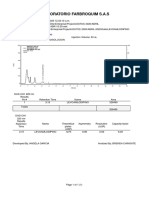
Chromatogram
No. Retention Time Peak Name Area
min mAU*min
1 5.68 BETAHISTINA 2HCl 3.363
Total: 3.363
SST Results
No.
Name Peak Name Peak Test Result Injection Inj. Eval. Result
1 Theoretical Plates (USP) BETAHISTINA 2HCl All Identified Peaks Passed D1 LOTE LP-LEF-19-002 4156.000
2 Peak Asymmetry BETAHISTINA 2HCl All Identified Peaks Failed 6 LOTE LP-LEF-19-00 0.923
BETAHISTINA 2HCl 1 LOTE LP-LEF-19-00 0.944
3 Resolution (USP) BETAHISTINA 2HCl All Identified Peaks NA -> Passed 1 LOTE LP-LEF-19-00 n.a.
4 Signal Noise n.a. Passed 1 LOTE LP-LEF-19-00 0.023
5 Signal / Noise Ratio BETAHISTINA 2HCl All Identified Peaks NA -> Passed 1 LOTE LP-LEF-19-00 n.a.
Number of executed test cases: 5 Total Result: Failed
Método creado por: Angela Garcia Analizado por: Lady Martinez
Chromeleon (c) Dionex
BETAHISTINA DICLORHIDRATO AGO-31-20/Integration Version 7.2.10.23925
Instrument:MP-15 Sequence:BETAHISTINA DICLORHIDRATO AGO-31-20 Page 2 of 6
LABORATORIO FARBROQUIM S.A.S
Injection Date/Time: 01/Sep/20 03:51
Processing Method: BETAHISTINA DICLORHIDRATO AGO-31-20
Instrument name: MP-15 Injection Type: Unknown
Injection Name: 01/Sep/2020 03:51 Injection Volume: 25.00
Comment: LEFLUX 8 mg TAB EN T3 TRILAMINADO
Directory: Instrument Data\MP-15\AGOSTO 2020
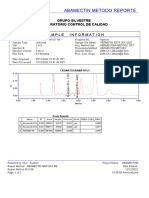
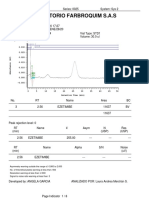
Chromatogram
No. Retention Time Peak Name Area
min mAU*min
1 5.68 BETAHISTINA 2HCl 3.290
Total: 3.290
SST Results
No.
Name Peak Name Peak Test Result Injection Inj. Eval. Result
1 Theoretical Plates (USP) BETAHISTINA 2HCl All Identified Peaks Passed D2 LOTE LP-LEF-19-002 3903.000
2 Peak Asymmetry BETAHISTINA 2HCl All Identified Peaks Failed 1 LOTE LP-LEF-19-00 0.944
BETAHISTINA 2HCl 2 LOTE LP-LEF-19-00 0.919
3 Resolution (USP) BETAHISTINA 2HCl All Identified Peaks NA -> Passed 2 LOTE LP-LEF-19-00 n.a.
4 Signal Noise n.a. Passed 2 LOTE LP-LEF-19-00 0.022
5 Signal / Noise Ratio BETAHISTINA 2HCl All Identified Peaks Passed 2 LOTE LP-LEF-19-00 1093.091
Number of executed test cases: 5 Total Result: Failed
Método creado por: Angela Garcia Analizado por: Lady Martinez
Chromeleon (c) Dionex
BETAHISTINA DICLORHIDRATO AGO-31-20/Integration Version 7.2.10.23925
Instrument:MP-15 Sequence:BETAHISTINA DICLORHIDRATO AGO-31-20 Page 3 of 6
LABORATORIO FARBROQUIM S.A.S
Injection Date/Time: 01/Sep/20 04:12
Processing Method: BETAHISTINA DICLORHIDRATO AGO-31-20
Instrument name: MP-15 Injection Type: Unknown
Injection Name: 01/Sep/2020 04:12 Injection Volume: 25.00
Comment: LEFLUX 8 mg TAB EN T3 TRILAMINADO
Directory: Instrument Data\MP-15\AGOSTO 2020
Chromatogram
No. Retention Time Peak Name Area
min mAU*min
1 5.68 BETAHISTINA 2HCl 3.399
Total: 3.399
SST Results
No.
Name Peak Name Peak Test Result Injection Inj. Eval. Result
1 Theoretical Plates (USP) BETAHISTINA 2HCl All Identified Peaks Passed D3 LOTE LP-LEF-19-002 4161.000
2 Peak Asymmetry BETAHISTINA 2HCl All Identified Peaks Failed 2 LOTE LP-LEF-19-00 0.919
BETAHISTINA 2HCl 3 LOTE LP-LEF-19-00 0.931
3 Resolution (USP) BETAHISTINA 2HCl All Identified Peaks NA -> Passed 3 LOTE LP-LEF-19-00 n.a.
4 Signal Noise n.a. Passed 3 LOTE LP-LEF-19-00 0.024
5 Signal / Noise Ratio BETAHISTINA 2HCl All Identified Peaks Passed 3 LOTE LP-LEF-19-00 827.108
Number of executed test cases: 5 Total Result: Failed
Método creado por: Angela Garcia Analizado por: Lady Martinez
Chromeleon (c) Dionex
BETAHISTINA DICLORHIDRATO AGO-31-20/Integration Version 7.2.10.23925
Instrument:MP-15 Sequence:BETAHISTINA DICLORHIDRATO AGO-31-20 Page 4 of 6
LABORATORIO FARBROQUIM S.A.S
Injection Date/Time: 01/Sep/20 04:33
Processing Method: BETAHISTINA DICLORHIDRATO AGO-31-20
Instrument name: MP-15 Injection Type: Unknown
Injection Name: 01/Sep/2020 04:33 Injection Volume: 25.00
Comment: LEFLUX 8 mg TAB EN T3 TRILAMINADO
Directory: Instrument Data\MP-15\AGOSTO 2020
Chromatogram
No. Retention Time Peak Name Area
min mAU*min
1 5.70 BETAHISTINA 2HCl 3.382
Total: 3.382
SST Results
No.
Name Peak Name Peak Test Result Injection Inj. Eval. Result
1 Theoretical Plates (USP) BETAHISTINA 2HCl All Identified Peaks Passed D4 LOTE LP-LEF-19-002 4171.000
2 Peak Asymmetry BETAHISTINA 2HCl All Identified Peaks Failed 3 LOTE LP-LEF-19-00 0.931
BETAHISTINA 2HCl 4 LOTE LP-LEF-19-00 0.922
3 Resolution (USP) BETAHISTINA 2HCl All Identified Peaks NA -> Passed 4 LOTE LP-LEF-19-00 n.a.
4 Signal Noise n.a. Passed 4 LOTE LP-LEF-19-00 0.027
5 Signal / Noise Ratio BETAHISTINA 2HCl All Identified Peaks Passed 4 LOTE LP-LEF-19-00 1072.375
Number of executed test cases: 5 Total Result: Failed
Método creado por: Angela Garcia Analizado por: Lady Martinez
Chromeleon (c) Dionex
BETAHISTINA DICLORHIDRATO AGO-31-20/Integration Version 7.2.10.23925
Instrument:MP-15 Sequence:BETAHISTINA DICLORHIDRATO AGO-31-20 Page 5 of 6
LABORATORIO FARBROQUIM S.A.S
Injection Date/Time: 01/Sep/20 04:54
Processing Method: BETAHISTINA DICLORHIDRATO AGO-31-20
Instrument name: MP-15 Injection Type: Unknown
Injection Name: 01/Sep/2020 04:54 Injection Volume: 25.00
Comment: LEFLUX 8 mg TAB EN T3 TRILAMINADO
Directory: Instrument Data\MP-15\AGOSTO 2020
Chromatogram
No. Retention Time Peak Name Area
min mAU*min
1 5.68 BETAHISTINA 2HCl 3.275
Total: 3.275
SST Results
No.
Name Peak Name Peak Test Result Injection Inj. Eval. Result
1 Theoretical Plates (USP) BETAHISTINA 2HCl All Identified Peaks Passed D5 LOTE LP-LEF-19-002 4132.000
2 Peak Asymmetry BETAHISTINA 2HCl All Identified Peaks Failed 4 LOTE LP-LEF-19-00 0.922
BETAHISTINA 2HCl 5 LOTE LP-LEF-19-00 0.932
3 Resolution (USP) BETAHISTINA 2HCl All Identified Peaks NA -> Passed 5 LOTE LP-LEF-19-00 n.a.
4 Signal Noise n.a. Passed 5 LOTE LP-LEF-19-00 0.018
5 Signal / Noise Ratio BETAHISTINA 2HCl All Identified Peaks Passed 5 LOTE LP-LEF-19-00 950.412
Number of executed test cases: 5 Total Result: Failed
Método creado por: Angela Garcia Analizado por: Lady Martinez
Chromeleon (c) Dionex
BETAHISTINA DICLORHIDRATO AGO-31-20/Integration Version 7.2.10.23925
Instrument:MP-15 Sequence:BETAHISTINA DICLORHIDRATO AGO-31-20 Page 6 of 6
LABORATORIO FARBROQUIM S.A.S
Injection Date/Time: 01/Sep/20 05:15
Processing Method: BETAHISTINA DICLORHIDRATO AGO-31-20
Instrument name: MP-15 Injection Type: Unknown
Injection Name: 01/Sep/2020 05:15 Injection Volume: 25.00
Comment: LEFLUX 8 mg TAB EN T3 TRILAMINADO
Directory: Instrument Data\MP-15\AGOSTO 2020
Chromatogram
No. Retention Time Peak Name Area
min mAU*min
1 5.68 BETAHISTINA 2HCl 3.379
Total: 3.379
SST Results
No.
Name Peak Name Peak Test Result Injection Inj. Eval. Result
1 Theoretical Plates (USP) BETAHISTINA 2HCl All Identified Peaks Passed D6 LOTE LP-LEF-19-002 4027.000
2 Peak Asymmetry BETAHISTINA 2HCl All Identified Peaks Failed 5 LOTE LP-LEF-19-00 0.932
BETAHISTINA 2HCl 6 LOTE LP-LEF-19-00 0.924
3 Resolution (USP) BETAHISTINA 2HCl All Identified Peaks NA -> Passed 6 LOTE LP-LEF-19-00 n.a.
4 Signal Noise n.a. Passed 6 LOTE LP-LEF-19-00 0.027
5 Signal / Noise Ratio BETAHISTINA 2HCl All Identified Peaks Passed 6 LOTE LP-LEF-19-00 507.866
Number of executed test cases: 5 Total Result: Failed
Método creado por: Angela Garcia Analizado por: Lady Martinez
Chromeleon (c) Dionex
BETAHISTINA DICLORHIDRATO AGO-31-20/Integration Version 7.2.10.23925
You might also like
- Self Diagnostics DTCS: Back To ArticleDocument41 pagesSelf Diagnostics DTCS: Back To ArticleNiculescuNo ratings yet
- Parametric Airfoil CatalogDocument570 pagesParametric Airfoil Catalogbrufpot0% (1)
- Heat Loss Insulated Pipe SpreadsheetDocument14 pagesHeat Loss Insulated Pipe SpreadsheetHernan RodriguezNo ratings yet
- Emergency ShutingdownDocument11 pagesEmergency ShutingdownOsama OmayerNo ratings yet
- Thermography Report-02 - 147Document5 pagesThermography Report-02 - 147madhavanNo ratings yet
- JW TICO Product Guide Issue 1-18 PDFDocument48 pagesJW TICO Product Guide Issue 1-18 PDFgkdora574No ratings yet
- Fault DiagnosisDocument98 pagesFault DiagnosisriajulNo ratings yet
- High Efficiency RF and Microwave Solid State Power AmplifiersFrom EverandHigh Efficiency RF and Microwave Solid State Power AmplifiersRating: 1 out of 5 stars1/5 (1)
- Equipment DatasheetDocument9 pagesEquipment DatasheetBimal DeyNo ratings yet
- Cispr18-2 (Ed2 0) enDocument76 pagesCispr18-2 (Ed2 0) enSajjad PirzadaNo ratings yet
- DML Parts CatalogDocument628 pagesDML Parts Catalog11173% (11)
- Onkyo HT r391Document82 pagesOnkyo HT r391Eustalio PelasNo ratings yet
- Monocrystalline Silicon PDFDocument5 pagesMonocrystalline Silicon PDFAdamRaczNo ratings yet
- Fire Monitor Data Sheet - 3-3Document1 pageFire Monitor Data Sheet - 3-3Bagus PrambudiNo ratings yet
- Result 2149Document2 pagesResult 2149LUIS STEVEN SANTISTEBAN OBREGONNo ratings yet
- Solvente 1Document1 pageSolvente 1Israel José MendozaNo ratings yet
- TG1 - 7UM621 V4 - 7 - PRN - 10 - 02 - 31Document14 pagesTG1 - 7UM621 V4 - 7 - PRN - 10 - 02 - 31PABITRA PATRANo ratings yet
- CTP, CTQ Check ListDocument177 pagesCTP, CTQ Check ListMd. Shahariar Imroz LinkonNo ratings yet
- Result 2151Document2 pagesResult 2151LUIS STEVEN SANTISTEBAN OBREGONNo ratings yet
- Trauma Male A: General Surgery: Team I Dr. Tamani/Lingan/Aguisanda/Baculi/Casco/ BulusanDocument11 pagesTrauma Male A: General Surgery: Team I Dr. Tamani/Lingan/Aguisanda/Baculi/Casco/ BulusanLuis PadillaNo ratings yet
- Thermography Report-03 - 04Document5 pagesThermography Report-03 - 04madhavanNo ratings yet
- TG2 - 7UM621 V4 - 7 - PRN - 10 - 13 - 56Document14 pagesTG2 - 7UM621 V4 - 7 - PRN - 10 - 13 - 56PABITRA PATRANo ratings yet
- Laboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Sens 5Document3 pagesLaboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Sens 5Brendapaez3No ratings yet
- Acura TSX 2004 PGM-FI SystemDocument200 pagesAcura TSX 2004 PGM-FI Systemjorge antonio guillenNo ratings yet
- Irina A Atas: List Pasien 29/11/19Document22 pagesIrina A Atas: List Pasien 29/11/19Jesiandra isabel M WagiuNo ratings yet
- Department of Biochemistry: Test Name Result Unit Bio. Ref. Range MethodDocument1 pageDepartment of Biochemistry: Test Name Result Unit Bio. Ref. Range MethodTomble BravoNo ratings yet
- Irina A Atas: List Pasien 29/11/19Document19 pagesIrina A Atas: List Pasien 29/11/19Jesiandra isabel M WagiuNo ratings yet
- Nittan Company, Limited: International DepartmentDocument5 pagesNittan Company, Limited: International DepartmentQuo QingNo ratings yet
- Trauma Male A: General Surgery: Team I Dr. Tamani/Lingan/Aguisanda/Baculi/Casco/ BulusanDocument21 pagesTrauma Male A: General Surgery: Team I Dr. Tamani/Lingan/Aguisanda/Baculi/Casco/ BulusanLuis PadillaNo ratings yet
- Fase Movil 2Document1 pageFase Movil 2Israel José MendozaNo ratings yet
- Hidrosin Plus Pleg X 12 STCK Lote 19258 FBQ122-PT0119 005Document20 pagesHidrosin Plus Pleg X 12 STCK Lote 19258 FBQ122-PT0119 005Brendapaez3No ratings yet
- Book 44 Answer KeyDocument17 pagesBook 44 Answer Keyyuvraj brahmbhattNo ratings yet
- DDR N°02 - Sydnw1 - Enf57 - 24122019Document3 pagesDDR N°02 - Sydnw1 - Enf57 - 24122019Kenaouia Bahaa100% (1)
- 4MVA - TRAFO - 7SJ663 V4 - 3 - PRN - 11 - 49 - 49Document10 pages4MVA - TRAFO - 7SJ663 V4 - 3 - PRN - 11 - 49 - 49PABITRA PATRANo ratings yet
- Nord Piano 4 Factory Program Presets: General NotesDocument20 pagesNord Piano 4 Factory Program Presets: General NotesSusskinoNo ratings yet
- Trauma Male A: General Surgery: Team I Dr. Tamani/Lingan/Aguisanda/Baculi/Casco/ BulusanDocument23 pagesTrauma Male A: General Surgery: Team I Dr. Tamani/Lingan/Aguisanda/Baculi/Casco/ BulusanLuis PadillaNo ratings yet
- GTA Turbo Blower Fire Alarm System LayoutDocument2 pagesGTA Turbo Blower Fire Alarm System Layoutchida mohaNo ratings yet
- Alba Report - CompressedDocument30 pagesAlba Report - Compressedwinston11No ratings yet
- Cromatograma HPLCDocument37 pagesCromatograma HPLCDarwin Andrés Taboada MartínezNo ratings yet
- Fase Movil 1Document1 pageFase Movil 1Israel José MendozaNo ratings yet
- Laboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Sens 5Document6 pagesLaboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Sens 5Brendapaez3No ratings yet
- Raport Sol D - Proba Necunoscuta - Screening Si Dozare UPLC-PDADocument1 pageRaport Sol D - Proba Necunoscuta - Screening Si Dozare UPLC-PDAMarianna MaryanaNo ratings yet
- Chromatogram and Results: Injection DetailsDocument5 pagesChromatogram and Results: Injection DetailsNova SeptiNo ratings yet
- SCRN20042412226258CN710Document1 pageSCRN20042412226258CN710sukamal.guhaNo ratings yet
- Safety Data Sheet: SECTION 1: Identification of The Substance/mixture and of The Company/ UndertakingDocument9 pagesSafety Data Sheet: SECTION 1: Identification of The Substance/mixture and of The Company/ UndertakingEmiro Jose Perez RodriguezNo ratings yet
- Hds Napa Mac's White Lithium Grease AerosolDocument6 pagesHds Napa Mac's White Lithium Grease Aerosolitzamar cruzNo ratings yet
- MotoroidDocument2 pagesMotoroidralim8254No ratings yet
- Test Report: Softlines Wastewater TestingDocument15 pagesTest Report: Softlines Wastewater TestingMusaNo ratings yet
- Ball Mill ToolsDocument2 pagesBall Mill ToolsCarlos Rafael Uriarte VasquezNo ratings yet
- 112T2395 D Device Summary AlarmDocument236 pages112T2395 D Device Summary AlarmJavier Cruz VargasNo ratings yet
- Trouble Codes BMW z4 E85Document16 pagesTrouble Codes BMW z4 E85mikailvalvesNo ratings yet
- Fase Movil 3Document1 pageFase Movil 3Israel José MendozaNo ratings yet
- Annexure - XXIV: Advance Claim Request Form Date:-05/10/2018Document3 pagesAnnexure - XXIV: Advance Claim Request Form Date:-05/10/2018dilip jotavaNo ratings yet
- Magnetic Flowmeter FT 103Document3 pagesMagnetic Flowmeter FT 103riswan nugrahaNo ratings yet
- Material Safety Data SheetDocument7 pagesMaterial Safety Data SheetAnonymous NgcpLQiNo ratings yet
- Prueba de EficienciaDocument11 pagesPrueba de EficienciaIván Andrés Benavides pajoyNo ratings yet
- No. Device Name Merk Tipe Serial Number: Daftar Inventaris AlatDocument6 pagesNo. Device Name Merk Tipe Serial Number: Daftar Inventaris AlatLevi AliciaNo ratings yet
- Trauma Male A: General Surgery: Team I Dr. Tamani/Lingan/Aguisanda/Baculi/Casco/ BulusanDocument12 pagesTrauma Male A: General Surgery: Team I Dr. Tamani/Lingan/Aguisanda/Baculi/Casco/ BulusanLuis PadillaNo ratings yet
- Final Payment / Reimbursment Voucher For Supply of Mi SystemDocument7 pagesFinal Payment / Reimbursment Voucher For Supply of Mi Systemdilip jotavaNo ratings yet
- DDR N°40 Omf-50 TP202 Du 05092021Document3 pagesDDR N°40 Omf-50 TP202 Du 05092021Mohamed SouidiNo ratings yet
- Report of Analysis: Sample Code Weight (KG) NO CV IM ASH VM FC TM TSDocument1 pageReport of Analysis: Sample Code Weight (KG) NO CV IM ASH VM FC TM TSTKM PKYNo ratings yet
- Trauma Male A: General Surgery: Team I Dr. Tamani/Lingan/Aguisanda/Baculi/Casco/ BulusanDocument23 pagesTrauma Male A: General Surgery: Team I Dr. Tamani/Lingan/Aguisanda/Baculi/Casco/ BulusanLuis PadillaNo ratings yet
- CANDEPREX A 16 MG TABLETA LOTE 170293 (STD + MTA AMLODIPINO)Document10 pagesCANDEPREX A 16 MG TABLETA LOTE 170293 (STD + MTA AMLODIPINO)Brendapaez3No ratings yet
- 1P-LSD Analytical ReportDocument6 pages1P-LSD Analytical ReportanastasiaNo ratings yet
- FP1021 D8VDocument6 pagesFP1021 D8VLaura SpearsNo ratings yet
- 8 Final Test - ACCU Sub F & Far F - MDocument1 page8 Final Test - ACCU Sub F & Far F - MLê Hồng SơnNo ratings yet
- Laboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Inhib Inhib Sens 3Document6 pagesLaboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Inhib Inhib Sens 3Brendapaez3No ratings yet
- Laboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Sens 5Document6 pagesLaboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Sens 5Brendapaez3No ratings yet
- Laboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Inhib Inhib Sens 3Document5 pagesLaboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Inhib Inhib Sens 3Brendapaez3No ratings yet
- Laboratorio Farbroquim S.A.S: 1400 1200 1000 800 600 400 200 0 0 2 4 6 8 10 12 14 16 18 20 22 24 Retention Time (Min)Document5 pagesLaboratorio Farbroquim S.A.S: 1400 1200 1000 800 600 400 200 0 0 2 4 6 8 10 12 14 16 18 20 22 24 Retention Time (Min)Brendapaez3No ratings yet
- Laboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Sens 5Document3 pagesLaboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Sens 5Brendapaez3No ratings yet
- CANDEPREX A 16 MG TABLETA LOTE 170293 (STD + MTA AMLODIPINO)Document10 pagesCANDEPREX A 16 MG TABLETA LOTE 170293 (STD + MTA AMLODIPINO)Brendapaez3No ratings yet
- ALENCAL IRBE 2.5 300 MG TABLETAS LOTE 16009 ESTANDARDocument7 pagesALENCAL IRBE 2.5 300 MG TABLETAS LOTE 16009 ESTANDARBrendapaez3No ratings yet
- ENSAYO DUOLIOP IMPUREZAS SIMVASTATINAaDocument10 pagesENSAYO DUOLIOP IMPUREZAS SIMVASTATINAaBrendapaez3No ratings yet
- ALENCAL IRBE 2.5 300 MG TABLETAS LOTE 16009 IRBESARTAN-1Document8 pagesALENCAL IRBE 2.5 300 MG TABLETAS LOTE 16009 IRBESARTAN-1Brendapaez3No ratings yet
- Hidrosin Plus Pleg X 12 STCK Lote 19258 FBQ122-PT0119 005Document20 pagesHidrosin Plus Pleg X 12 STCK Lote 19258 FBQ122-PT0119 005Brendapaez3No ratings yet
- Impurezas Ezetimibe Ene-31-20Document10 pagesImpurezas Ezetimibe Ene-31-20Brendapaez3No ratings yet
- Impurezas Ezetimibe Ene-29-20Document9 pagesImpurezas Ezetimibe Ene-29-20Brendapaez3No ratings yet
- Laboratorio Farbroquim S.A.S: N-Meth 0 Inhib Sens 3Document8 pagesLaboratorio Farbroquim S.A.S: N-Meth 0 Inhib Sens 3Brendapaez3No ratings yet
- Estandar Disolucion LevoDocument5 pagesEstandar Disolucion LevoBrendapaez3No ratings yet
- Sustancias RelacionadasDocument6 pagesSustancias RelacionadasBrendapaez3No ratings yet
- Laboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Inhib Inhib Sens 3Document5 pagesLaboratorio Farbroquim S.A.S: N-Meth 0 Inhib Inhib Inhib Inhib Sens 3Brendapaez3No ratings yet
- DAD-CH1 273 NM Results: Retention Time Name AreaDocument9 pagesDAD-CH1 273 NM Results: Retention Time Name AreaBrendapaez3No ratings yet
- Workman 2017 A Review of Calibration Transfer Practices and Instrument Differences in SpectrosDocument26 pagesWorkman 2017 A Review of Calibration Transfer Practices and Instrument Differences in SpectrosoalegionNo ratings yet
- Edexcel IGCSE Unit 2E Homeostasis and Excretion - Self-Assessment SheetDocument6 pagesEdexcel IGCSE Unit 2E Homeostasis and Excretion - Self-Assessment SheetAli ALEBRAHIMNo ratings yet
- Chapter 27 Analysis Edexcel Igcse Chemistry ReviewDocument14 pagesChapter 27 Analysis Edexcel Igcse Chemistry ReviewRafid Al NahiyanNo ratings yet
- Ertl MGT 4010 Final Exam APPLICATION Semester 2 2019Document6 pagesErtl MGT 4010 Final Exam APPLICATION Semester 2 2019Raihah Nabilah HashimNo ratings yet
- Aptitude TestDocument6 pagesAptitude TestMeera SeshannaNo ratings yet
- Aggregate Crushing ValueDocument8 pagesAggregate Crushing ValueEngineeri TadiyosNo ratings yet
- Module 4: Plant Growth Factors and Latest Trend in Crop ScienceDocument26 pagesModule 4: Plant Growth Factors and Latest Trend in Crop ScienceNorhanah Dionisio BarubaNo ratings yet
- Datacamp Python 4Document37 pagesDatacamp Python 4Luca FarinaNo ratings yet
- STM32 HTTP CameraDocument8 pagesSTM32 HTTP Cameraengin kavakNo ratings yet
- Polymer Science and Technology: This Polymerization Yields AnDocument20 pagesPolymer Science and Technology: This Polymerization Yields AnakhilNo ratings yet
- Fractal PDFDocument9 pagesFractal PDFMukundNo ratings yet
- MAPEH (Arts) : Quarter 2Document40 pagesMAPEH (Arts) : Quarter 2Eiay CommsNo ratings yet
- PWV3Document36 pagesPWV3Tony AppsNo ratings yet
- Global Intrima Bulletin - Fire Protection MaintenanceDocument2 pagesGlobal Intrima Bulletin - Fire Protection MaintenancewawanNo ratings yet
- Speed PDFDocument1 pageSpeed PDFhendrik koesumaNo ratings yet
- Cinema - Problem DescriptionDocument10 pagesCinema - Problem DescriptionVladaNo ratings yet
- Vedic Maths 1Document5 pagesVedic Maths 1Ajmal AshrafNo ratings yet
- Abstracts About WeldingDocument9 pagesAbstracts About WeldingVinayak BhustalimathNo ratings yet
- Magnetic and Gravity Toolface and How To Interpret The Meaning For Directional Drilling - PDFDocument17 pagesMagnetic and Gravity Toolface and How To Interpret The Meaning For Directional Drilling - PDFHeberth DesbloqueoNo ratings yet
- Gozak - LeonidovDocument14 pagesGozak - LeonidovMartí ARNo ratings yet
- Tutorial Sheets Unit II, III, AND IV MEC 135Document9 pagesTutorial Sheets Unit II, III, AND IV MEC 135Rakesh AmaragondaNo ratings yet
- Turck Foundation Fieldbus JunctionsDocument33 pagesTurck Foundation Fieldbus JunctionsJose NavarreteNo ratings yet
- Computer Literacy - Lesson 1 - Computer SystemsDocument1 pageComputer Literacy - Lesson 1 - Computer SystemscuddlesttNo ratings yet
- Sas ViyaDocument4 pagesSas ViyaRocío VázquezNo ratings yet