Professional Documents
Culture Documents
Chapter 8 Evaporation-Exercise
Uploaded by
Nguyễn QuangCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 8 Evaporation-Exercise
Uploaded by
Nguyễn QuangCopyright:
Available Formats
Exercise 1
A single effect evaporator is required to concentrate a solution from 10% solids to 30% solids at the rate of 250kg
of feed per hour. If the pressure in the evaporator is 77kPa absolute, and if steam is available at 300kPa, calculate
the quantity of steam required per hour and the area of heat transfer surface if the overall heat transfer
coefficient is 1700Wm-2.oC-1
Assume that the temperature of the feed is 18oC and that the boiling point of the solution under the pressure of
77kPa absolute is 91oC. Assume, also, that the specific heat of the solution is the same as for water, that is 4.186
kJkg-1oC-1, and the latent heat of vaporization of the solution is the same as that for water under the same
conditions.
Calculate steam usage and heat transfer surface.
TS. Nguyễn Thị Lê Liên_BM QTTB_Khoa KTHH_ĐHBK
Exercise 2
A single effect evaporation system to concentrate 2 ton/h caustic solution
with initial concentration 14.1% (weight) to 24.1% (w). The steam is
saturated at 1500C, condensate is in saturated state. The vapor is at
atmospheric pressure. The heat loss of the equipment is 58,000W.
Calculate the steam consumption used for the following cases
a. Feed solution at 200C.
b. Feed solution at boiling point
c. Feed solution at 1300C
TS. Nguyễn Thị Lê Liên_BM QTTB_Khoa KTHH_ĐHBK
Exercise 3
A continuous single-effect evaporator concentrates 9072 kg/h of a 1.0 wt % salt
solution entering at 38ºC to a final concentration of 1.5 wt %.
The vapor space of the evaporator is at 101.325 kPa (1.0 atm abs) and the steam
supplied is saturated at 150 kPa. The overall coefficient U = 1704 W/m2.K.
Calculate the amounts of vapor and liquid products and the heat- transfer area
required. Assumed that, since it its dilute, the solution has the same boiling point
as water.
TS. Nguyễn Thị Lê Liên_BM QTTB_Khoa KTHH_ĐHBK
Exercise 4
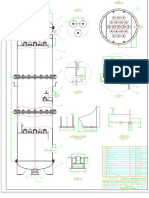
• A type 1-1 shell-tube condenser condenses 5000 kg/h of saturated ethanol vapor
at normal pressure with cooling water at 25°C. Liquid ethanol exits the apparatus
at a temperature of 600°C. The water leaving the device has a temperature of
400C. Let the heat coefficient of steam side is 6000W/m2.K, liquid ethanol side is
3000W/m2K, water side is 2000W/m2K. Let's:
a) Draw device diagram, line symbol
b) Calculate the heat transfer coefficient of the device.
c) Calculate the amount of water needed to cool down
d) Calculate the number of pipes 21x2mm if L= 50 is selected.
TS. Nguyễn Thị Lê Liên_BM QTTB_Khoa KTHH_ĐHBK
Exercise 5
Use Babo method to calculate temperature elevation of CaCl2 25% (by
weight) at absolute pressure p0=0.36 kg/cm2.
The boiling point of CaCl2 25% at atmospheric pressure (1.033 kg/cm2) is
given at 107.50C, and at that temperature, the vapor pressure is 1.345
kg/cm2
TS. Nguyễn Thị Lê Liên_BM QTTB_Khoa KTHH_ĐHBK
Exercise 6- condensers
How much water would be required in a jet condenser to condense the vapours
from an evaporator evaporating 5000kgh-1 of water under a pressure of 15cm Hg?
The condensing water is available at 18oC and the highest allowable temperature
for water discharged from the condenser is 35oC
TS. Nguyễn Thị Lê Liên_BM QTTB_Khoa KTHH_ĐHBK
Exercise 7-multi-effect
Estimate the requirements of steam and heat transfer surface, and the evaporating
temperatures in each effect, for a triple effect evaporator evaporating 500 kgh-1 of a
10% solution up to a 30% solution. Steam is available at 200kPa gauge and the
pressure in the evaporation space in the final effect is 60kPa absolute. Assume that
the overall heat transfer coefficients are 2270, 2000 and 1420Wm-2oC in the first,
second and third effects, respectively. Neglect sensible heat. Assume no boiling-
point elevation, and also equal heat transfer in each effect
TS. Nguyễn Thị Lê Liên_BM QTTB_Khoa KTHH_ĐHBK
You might also like
- Asignment - Chapter 2Document9 pagesAsignment - Chapter 2Nguyễn Đạt100% (1)
- Introduction to the Main Branches of ChemistryDocument3 pagesIntroduction to the Main Branches of ChemistryGiang Nguyen Thi HuongNo ratings yet
- Final Exam - 2017 PDFDocument7 pagesFinal Exam - 2017 PDFOlla 8352No ratings yet
- BÁO CÁO THÍ NGHIỆM HÓA LYDocument23 pagesBÁO CÁO THÍ NGHIỆM HÓA LYNhat Quang PhanNo ratings yet
- The Fischer Esterification of BenzocaineDocument5 pagesThe Fischer Esterification of BenzocaineMikeNo ratings yet
- Group 1 - ETHYLBENZENE PRODUCTIONDocument7 pagesGroup 1 - ETHYLBENZENE PRODUCTIONQuỳnh Như PhạmNo ratings yet
- Part 5 Test 3Document3 pagesPart 5 Test 3TIEN NGUYEN HOANG THUYNo ratings yet
- Designing a 4 Tons/H Tomato Paste EvaporatorDocument92 pagesDesigning a 4 Tons/H Tomato Paste EvaporatorNhư NguyễnNo ratings yet
- MSDS & TDS - Masterseal 505 PDFDocument2 pagesMSDS & TDS - Masterseal 505 PDFengsam777100% (1)
- Real InDictionary of Chemistry 4 BookDocument33 pagesReal InDictionary of Chemistry 4 Booktire farrokhzadNo ratings yet
- Science of States of MatterDocument28 pagesScience of States of Mattercecil tayagNo ratings yet
- Bài tập Hoá lý 2 (Physical Chemistry 2 - Homework)Document10 pagesBài tập Hoá lý 2 (Physical Chemistry 2 - Homework)anhxuan03102001No ratings yet
- INTERPHASE MASS TRANSFERDocument58 pagesINTERPHASE MASS TRANSFERParitosh Chaudhary0% (1)
- P740JDocument2 pagesP740JTan Chen TatNo ratings yet
- Experimental Report of Unit 2Document13 pagesExperimental Report of Unit 2Quốc Thắng Nguyễn100% (1)
- Bài tập Hoá lý 1 (Physical Chemistry 1 - Homework)Document13 pagesBài tập Hoá lý 1 (Physical Chemistry 1 - Homework)Minh ThưNo ratings yet
- Các dạng đề thi Anh văn B2/Anh văn 3 (theo hình thức trực tuyếnDocument14 pagesCác dạng đề thi Anh văn B2/Anh văn 3 (theo hình thức trực tuyếnTuyền Nguyễn100% (1)
- ViscositiesDocument5 pagesViscosities12 Hóa0% (1)
- Abstracts Vol 5Document168 pagesAbstracts Vol 5siriuslotNo ratings yet
- Chapter 1 and 2 AssignmentsDocument24 pagesChapter 1 and 2 AssignmentsHà Thế VinhNo ratings yet
- Impact StrengthDocument13 pagesImpact StrengthManishUpadhyay100% (1)
- CHU DE 3 - PHAN 1-Chat Nhu Hoa - R3Document93 pagesCHU DE 3 - PHAN 1-Chat Nhu Hoa - R3Hoài Thương0% (1)
- Process Parameters and Production Line for Tofu ManufacturingDocument12 pagesProcess Parameters and Production Line for Tofu ManufacturingAnuj Singh100% (1)
- Consolidation B2: Pg. 1 Ms H NHDocument3 pagesConsolidation B2: Pg. 1 Ms H NHTrương Ngọc Vĩnh Khang100% (1)
- Hướng Dẫn Bài Tập Hoá Đại Cương 2Document56 pagesHướng Dẫn Bài Tập Hoá Đại Cương 2Thái BảoNo ratings yet
- Page 1 of 4Document4 pagesPage 1 of 4ShashwatAgarwalNo ratings yet
- Recommended Soil Organic Matter TestsDocument9 pagesRecommended Soil Organic Matter TestsgagileNo ratings yet
- IOCCC Analytical Methods GuideDocument1 pageIOCCC Analytical Methods GuideTamilarasan ArasurNo ratings yet
- BT tuần 2Document2 pagesBT tuần 2Đặng NhungNo ratings yet
- DNS A InstructionsDocument2 pagesDNS A InstructionsWilda PanjaitanNo ratings yet
- Determining Molar Mass Using CryosDocument6 pagesDetermining Molar Mass Using CryosValentin-AngeloUzunovNo ratings yet
- ISO 17025 Accredited Mechanical Testing LaboratoryDocument2 pagesISO 17025 Accredited Mechanical Testing LaboratoryAnish KumarNo ratings yet
- Shell and tube heat exchanger design and analysisDocument3 pagesShell and tube heat exchanger design and analysisHoài ThươngNo ratings yet
- Formalreport INVERTASEDocument2 pagesFormalreport INVERTASEyamsytron50% (4)
- Density of KOH SolutionsDocument1 pageDensity of KOH SolutionsjohnihaasNo ratings yet
- Exercises Part 1Document5 pagesExercises Part 1Le Thai SonNo ratings yet
- Bài Tập Phân Tích Công CụDocument38 pagesBài Tập Phân Tích Công Cụ12a50% (1)
- Blood test analysis formula interpretationDocument11 pagesBlood test analysis formula interpretationTùng HuynhNo ratings yet
- HW 5Document2 pagesHW 5msoccerdude291No ratings yet
- 7acids and Other Products of Oxidation of SugarsDocument108 pages7acids and Other Products of Oxidation of SugarsHung le Van100% (1)
- Simple Present and Gerunds ReviewDocument1 pageSimple Present and Gerunds ReviewJaqueline Zamorano PèrezNo ratings yet
- Pca-phân tích thành phần chínhDocument30 pagesPca-phân tích thành phần chínhChemTuongViNo ratings yet
- Le Ngoc Lieu - Lecture 1 - Chapter 1Document28 pagesLe Ngoc Lieu - Lecture 1 - Chapter 1lieu_hyacinthNo ratings yet
- Midterm Enzyme Fermentation 1Document20 pagesMidterm Enzyme Fermentation 1Thuỳ TrangNo ratings yet
- Analysis of Ethanol in Gasoline by Gas Chromatography and Infrared SpectrosDocument10 pagesAnalysis of Ethanol in Gasoline by Gas Chromatography and Infrared Spectrosapi-253994289No ratings yet
- Dr. David Benson's camshaft assembly instructionsDocument3 pagesDr. David Benson's camshaft assembly instructionsThanh Vo100% (1)
- LC method for benzoate, caffeine, and saccharin in beveragesDocument1 pageLC method for benzoate, caffeine, and saccharin in beveragesblink scientificNo ratings yet
- PS1 08-SolutionDocument7 pagesPS1 08-SolutionNguyễn Vũ Quang Thành100% (1)
- Homework 01 (Englis Proficiency 2)Document7 pagesHomework 01 (Englis Proficiency 2)AINA NABIHAHNo ratings yet
- Corn Chips Quick GuideDocument38 pagesCorn Chips Quick GuideHillary KabillahNo ratings yet
- Ethylene Oxidation On Silver Catalysts eDocument22 pagesEthylene Oxidation On Silver Catalysts ePaola GarciaNo ratings yet
- Factor Affecting Quality of Mozzarella CheeseDocument5 pagesFactor Affecting Quality of Mozzarella CheeseWei Ching TayNo ratings yet
- BÀI 6 THUỐC BỘT CỐMDocument44 pagesBÀI 6 THUỐC BỘT CỐMMinh Thông NguyễnNo ratings yet
- Bubble Cap Distillation ColumnDocument1 pageBubble Cap Distillation ColumnVinh Lê KhảiNo ratings yet
- Duoc Lieu Chua Anthranoid B7Document52 pagesDuoc Lieu Chua Anthranoid B7Tuấn Trương NgọcNo ratings yet
- Advanced Chemistry Homework SolutionsDocument4 pagesAdvanced Chemistry Homework SolutionsHuy TranNo ratings yet
- Practice Table Completion Question PDFDocument3 pagesPractice Table Completion Question PDFБат-Ирээдүй ЖаргалNo ratings yet
- Thu Isome HoaDocument71 pagesThu Isome HoaHoai Thuong NguyenNo ratings yet
- Charantin HPLCDocument4 pagesCharantin HPLCReymart SangalangNo ratings yet
- Chemical Engineering Technology 3B (Cmtb321) Evaporation Tutorial QuestionsDocument2 pagesChemical Engineering Technology 3B (Cmtb321) Evaporation Tutorial QuestionseyezakeyeNo ratings yet
- Pbi 12505Document16 pagesPbi 12505Nguyễn QuangNo ratings yet
- Carbohydrate CatabolismDocument4 pagesCarbohydrate CatabolismNguyễn QuangNo ratings yet
- GT Học Phần Kinh Tế Chính Trị MNL (K) Tr Đầu- Tr100Document98 pagesGT Học Phần Kinh Tế Chính Trị MNL (K) Tr Đầu- Tr100Duy AnhNo ratings yet
- Book Chapter Final Version Chapman Kent Corrected LFDocument16 pagesBook Chapter Final Version Chapman Kent Corrected LFNguyễn QuangNo ratings yet
- Catalase: Hans LuckDocument10 pagesCatalase: Hans LuckNguyễn QuangNo ratings yet
- Template PowerPoint - ViewSonic Projector 04Document28 pagesTemplate PowerPoint - ViewSonic Projector 04Nguyễn QuangNo ratings yet
- Acs Jafc 9b04385Document66 pagesAcs Jafc 9b04385Nguyễn QuangNo ratings yet
- Industrial-Scale Application of Enzymes To The Fats and OIl IndustryDocument3 pagesIndustrial-Scale Application of Enzymes To The Fats and OIl IndustryAndre Maxwel ManikNo ratings yet
- Application of Enzymes As Food Antioxidants: ReviewDocument5 pagesApplication of Enzymes As Food Antioxidants: ReviewNguyễn QuangNo ratings yet
- Template PowerPoint - ViewSonic Projector 05Document26 pagesTemplate PowerPoint - ViewSonic Projector 05Nguyễn QuangNo ratings yet
- Template PowerPoint - ViewSonic Projector 02Document26 pagesTemplate PowerPoint - ViewSonic Projector 02Nguyễn QuangNo ratings yet
- CAS - Digital MarketingDocument22 pagesCAS - Digital MarketingCA Pham KhaiNo ratings yet
- Template PowerPoint - ViewSonic Projector 06Document26 pagesTemplate PowerPoint - ViewSonic Projector 06Nguyễn QuangNo ratings yet
- Template PowerPoint - ViewSonic Projector 02Document26 pagesTemplate PowerPoint - ViewSonic Projector 02Nguyễn QuangNo ratings yet
- Template PowerPoint - ViewSonic Projector 10Document30 pagesTemplate PowerPoint - ViewSonic Projector 10Nguyễn QuangNo ratings yet
- CAS - Digital MarketingDocument22 pagesCAS - Digital MarketingCA Pham KhaiNo ratings yet
- Template PowerPoint - ViewSonic Projector 11Document26 pagesTemplate PowerPoint - ViewSonic Projector 11Nguyễn QuangNo ratings yet
- Foods 11 01388 v2Document18 pagesFoods 11 01388 v2Nguyễn QuangNo ratings yet
- Template PowerPoint - ViewSonic Projector 04Document28 pagesTemplate PowerPoint - ViewSonic Projector 04Nguyễn QuangNo ratings yet
- Industrial Biotechnology An OverviewDocument36 pagesIndustrial Biotechnology An OverviewAmeera Naeem ButtNo ratings yet
- TBP01x 1.3 SlidesDocument24 pagesTBP01x 1.3 SlidesNguyễn QuangNo ratings yet
- IELTS Task 1 New Answer SheetDocument2 pagesIELTS Task 1 New Answer SheetChrisGovasNo ratings yet
- Home Science Biology Difference Between Fermentation and Respiration Difference Between Fermentation and RespirationDocument12 pagesHome Science Biology Difference Between Fermentation and Respiration Difference Between Fermentation and RespirationNguyễn QuangNo ratings yet
- Sugar FermentationDocument1 pageSugar FermentationNguyễn QuangNo ratings yet
- Genes 13 00230Document21 pagesGenes 13 00230Nguyễn QuangNo ratings yet
- Carbohydrate CatabolismDocument4 pagesCarbohydrate CatabolismNguyễn QuangNo ratings yet
- Legras2007MolEcol With Cover Page v2Document13 pagesLegras2007MolEcol With Cover Page v2Nguyễn QuangNo ratings yet
- Bài giảng tuần 2Document51 pagesBài giảng tuần 2Nguyễn QuangNo ratings yet
- Bài giảng tuần 1Document43 pagesBài giảng tuần 1Nguyễn QuangNo ratings yet
- Rotavapor® R-100 Operation ManualDocument62 pagesRotavapor® R-100 Operation ManualCarla Sánchez GallardoNo ratings yet
- Car Hvac Systems: Mark Norman F. Chan Mark Paul C. PeñarroyoDocument34 pagesCar Hvac Systems: Mark Norman F. Chan Mark Paul C. PeñarroyoJohndelon P. MendozaNo ratings yet
- 657 Gb1064acDocument2 pages657 Gb1064acCarmen IoanaNo ratings yet
- 62-61118-04 Supra - 850 - 850MTDocument98 pages62-61118-04 Supra - 850 - 850MTJuan M Marín100% (1)
- Reportsheet#6 - Properties and Purification of Water - Chem1103lDocument7 pagesReportsheet#6 - Properties and Purification of Water - Chem1103lMarielleCaindecNo ratings yet
- Ast D 5236Document18 pagesAst D 5236Jesús GarcíaNo ratings yet
- Process Control Philosophy 05-01-23Document8 pagesProcess Control Philosophy 05-01-23svnaik14No ratings yet
- Refrigeration and Air Conditioning Components GuideDocument43 pagesRefrigeration and Air Conditioning Components GuideAshim Lamichhane100% (1)
- Battery Thermal Management System For Electric Vehicle Using Heat Pipes-Smith2018Document13 pagesBattery Thermal Management System For Electric Vehicle Using Heat Pipes-Smith2018Stacy WilsonNo ratings yet
- Section 1 - General Information: 1.1 Certification 1.2 Screw Compressor Size (Displacement)Document25 pagesSection 1 - General Information: 1.1 Certification 1.2 Screw Compressor Size (Displacement)EdmarNo ratings yet
- NED University Advanced Heat Transfer Exam QuestionsDocument1 pageNED University Advanced Heat Transfer Exam QuestionsHassan FaheemNo ratings yet
- Bas Svp047a en - 04012021Document40 pagesBas Svp047a en - 04012021adriano.caputiNo ratings yet
- Student's Technical Report on Industrial Training ExperienceDocument42 pagesStudent's Technical Report on Industrial Training ExperienceRichard Banks100% (1)
- Refrigeration and Heat Pump.. (GROUP-5) PDFDocument6 pagesRefrigeration and Heat Pump.. (GROUP-5) PDFmahirtajuar128No ratings yet
- Simple DistillationDocument5 pagesSimple DistillationJheian Christian TubleNo ratings yet
- Kcse Form 1 Chemistry NotesDocument117 pagesKcse Form 1 Chemistry NotesCaroline MugureNo ratings yet
- RC Group Brochure NEXT EVO DX Van DE WIT DatacenterkoelingDocument6 pagesRC Group Brochure NEXT EVO DX Van DE WIT Datacenterkoelingeep saepudinNo ratings yet
- 30MP 23siDocument8 pages30MP 23siFredy MurilloNo ratings yet
- Manual Destilador de CianurosDocument22 pagesManual Destilador de CianurosCarolinaNo ratings yet
- Preboards 2-PipeDocument4 pagesPreboards 2-PipeBenedictVillaminPolicarpioNo ratings yet
- HVACDocument26 pagesHVACAldrich GuarinNo ratings yet
- List of Chemistry Laboratory Apparatus and Their UsesDocument20 pagesList of Chemistry Laboratory Apparatus and Their Usesdexter timbanganNo ratings yet
- Alfa Laval USA Customer Service and Vapour Compression CycleDocument6 pagesAlfa Laval USA Customer Service and Vapour Compression CycleShoonNo ratings yet
- 1 Cond and EvapDocument27 pages1 Cond and Evaphussein9388hsNo ratings yet
- HARAMAYA INSTITUTE OF TECHNOLOGY REFRIGERATION AND AIR CONDITIONINGDocument108 pagesHARAMAYA INSTITUTE OF TECHNOLOGY REFRIGERATION AND AIR CONDITIONINGMoges AsefaNo ratings yet
- Refrigeration 1Document13 pagesRefrigeration 1Vishwanathan RishanthNo ratings yet
- Vapor Comprision DistillationDocument5 pagesVapor Comprision DistillationBAKRNo ratings yet
- How Window Air Conditioners WorkDocument11 pagesHow Window Air Conditioners WorkSAATVIK JAINNo ratings yet
- Steam Condensers and Cooling TowersDocument22 pagesSteam Condensers and Cooling Towersذال شراحبئيلNo ratings yet
- Voc ControlsDocument48 pagesVoc ControlsnarlaNo ratings yet