Professional Documents
Culture Documents
Dokumen PDF 27
Uploaded by
Farhan Farhan0 ratings0% found this document useful (0 votes)
9 views1 page1. The document describes a procedure where students add vanilla extract to one balloon and inflate it, then inflate a second balloon without vanilla as a control. They observe and record the temperatures of the balloons and how they change over 24 hours.
2. Students are instructed to analyze their observations and draw conclusions about how the vanilla evaporated and whether its odor escaped the balloon. They are asked to hypothesize and compare their hypothesis to Dalton's atomic theory.
3. Potential sources of error and factors affecting different groups' results are discussed. Students are asked questions about why helium balloons float longer and how industrial gases are stored.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document1. The document describes a procedure where students add vanilla extract to one balloon and inflate it, then inflate a second balloon without vanilla as a control. They observe and record the temperatures of the balloons and how they change over 24 hours.
2. Students are instructed to analyze their observations and draw conclusions about how the vanilla evaporated and whether its odor escaped the balloon. They are asked to hypothesize and compare their hypothesis to Dalton's atomic theory.
3. Potential sources of error and factors affecting different groups' results are discussed. Students are asked questions about why helium balloons float longer and how industrial gases are stored.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
9 views1 pageDokumen PDF 27
Uploaded by
Farhan Farhan1. The document describes a procedure where students add vanilla extract to one balloon and inflate it, then inflate a second balloon without vanilla as a control. They observe and record the temperatures of the balloons and how they change over 24 hours.
2. Students are instructed to analyze their observations and draw conclusions about how the vanilla evaporated and whether its odor escaped the balloon. They are asked to hypothesize and compare their hypothesis to Dalton's atomic theory.
3. Potential sources of error and factors affecting different groups' results are discussed. Students are asked questions about why helium balloons float longer and how industrial gases are stored.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
CHAPTER 4 CHEMLAB
Procedure Cleanup and Disposal
1. Using the medicine dropper, add 25 to 30 drops of 1. After the vanilla has dried, deflate the balloon by
vanilla extract to the first balloon. puncturing it with a sharp object.
2. Dispose of the pieces of the balloon as directed by
your teacher.
Analyze and Conclude
1. Observing and Inferring How did the relative
volumes of balloons 1 and 2 change after 24
hours? Explain.
2. Observing and Inferring By comparing the
relative temperatures of balloons 1 and 2, what can
you conclude about the temperature change as the
vanilla evaporated? Explain.
3. Observing and Inferring Did the vanilla’s odor
get outside the balloon and fill the enclosed space?
Explain.
4. Predicting Do you think vanilla will leak more
rapidly from a fully inflated balloon or from a half-
inflated balloon? Explain.
5. Hypothesizing Write a hypothesis that explains
2. Inflate the balloon so its walls are tightly stretched,
your observations.
but not stretched so tightly that the balloon is in dan-
ger of bursting. Try to keep the vanilla in one loca- 6. Comparing and Contrasting Compare your
tion as the balloon is inflated. Tie the balloon closed. hypothesis to Dalton’s atomic theory. In what ways
is it similar? How is it different?
3. Feel the outside of the balloon where the vanilla is
located and note the temperature of this area rela- 7. Error Analysis What factors might affect the
tive to the rest of the balloon. Record your obser- results of different groups that performed the
vations in the data table. experiment? What types of errors might have
occurred during the procedure?
4. Use only air to inflate a second balloon to approxi-
mately the same size as that of the first, and tie it
closed. Feel the outside of the second balloon. Real-World Chemistry
Make a relative temperature comparison to that of 1. Explain why helium-filled, Mylar-foil balloons can
the first balloon. Record your initial observations. float freely for several weeks, but latex balloons
5. Place the inflated balloons in a small, enclosed for less than 24 hours.
area such as a closet or student locker. 2. How are high-pressure gases stored for laboratory
6. The next day, repeat the observations in steps 3 and industrial use to prevent loss?
and 4 after the vanilla has dried inside the balloon.
Record these final observations.
7. To avoid splattering your clothes with dark brown
vanilla, do not deflate the balloon until the vanilla
has dried inside.
CHEMLAB 109
You might also like
- 10th-PHYSICS (EM) - Study Material For Slow Learners-SreekarDocument25 pages10th-PHYSICS (EM) - Study Material For Slow Learners-SreekarSarala Kakavakam100% (4)
- Grade 8 MATH 4th GradingDocument3 pagesGrade 8 MATH 4th Gradingrenz100% (1)
- Charle's Law (DLP)Document8 pagesCharle's Law (DLP)Marvin Eusebio100% (1)
- ZZ - Introduction To Lorentz Geometry Curves and Surfaces by Alexandre Lymberopoulos and Ivo Terek CoutoDocument351 pagesZZ - Introduction To Lorentz Geometry Curves and Surfaces by Alexandre Lymberopoulos and Ivo Terek Coutorajesh kumarNo ratings yet
- Module 4 Laboratory Safety PrecautionsDocument26 pagesModule 4 Laboratory Safety PrecautionsKaye Celyne C AmaproNo ratings yet
- 5 EsDocument3 pages5 EsMary Joselyn BodionganNo ratings yet
- 51 Rodney Y. Sharp Steps in Commutative Algebra 2001Document367 pages51 Rodney Y. Sharp Steps in Commutative Algebra 2001Trần Nam SơnNo ratings yet
- Boyles LawDocument3 pagesBoyles Lawaiza larrozaNo ratings yet
- Q4 Science 10 Week2Document3 pagesQ4 Science 10 Week2Edison Caringal50% (2)
- Gen Chem 2 Q2 Module 2Document24 pagesGen Chem 2 Q2 Module 2Bernalene TasiNo ratings yet
- 7es Lesson Plan in ScienceDocument4 pages7es Lesson Plan in ScienceFrancis Mae NabascaNo ratings yet
- Volcano Lesson Plan Grade 9Document4 pagesVolcano Lesson Plan Grade 9Erica De Vera89% (18)
- Final Revised Las in Science 9 q3w1Document5 pagesFinal Revised Las in Science 9 q3w1Ma.Kristine Ibarreta JazulNo ratings yet
- Ingénieur Polytechnicien Program Brochure 2022Document30 pagesIngénieur Polytechnicien Program Brochure 2022Estefânio FernandoNo ratings yet
- LP Boyle's LawDocument2 pagesLP Boyle's Lawxena marie sumadiaNo ratings yet
- Lesson Plan About MatterDocument4 pagesLesson Plan About MatterglaizaNo ratings yet
- Lesson Plan For Co 2Document5 pagesLesson Plan For Co 2Rycel Mae dela Torre100% (1)
- Lesson Plan - TocaDocument6 pagesLesson Plan - TocaAaron Asne100% (1)
- Lesson-Plan-Avogadro's LawDocument5 pagesLesson-Plan-Avogadro's LawQUEENY CORONELNo ratings yet
- DLL Week 7 FontillasDocument4 pagesDLL Week 7 Fontillasbren.abadNo ratings yet
- Cyclone Cyclone Experiment Junior FinalDocument3 pagesCyclone Cyclone Experiment Junior FinalcatigumfNo ratings yet
- Grades 1 To 12 Daily Lesson Log School Grade Level Teacher Learning Area Teaching Dates and Time QuarterDocument15 pagesGrades 1 To 12 Daily Lesson Log School Grade Level Teacher Learning Area Teaching Dates and Time QuarterImelda Pusta FrancisquiteNo ratings yet
- Atmosphere: Air andDocument20 pagesAtmosphere: Air andEren SevinceNo ratings yet
- Science 3 DLPDocument3 pagesScience 3 DLPCATHERINE FAJARDONo ratings yet
- JASMIN.D21-dlp With Attached Worksheets - Effect of Nature of Solute and Solvent in A SolutionDocument8 pagesJASMIN.D21-dlp With Attached Worksheets - Effect of Nature of Solute and Solvent in A SolutionAbigail JasminNo ratings yet
- Daily Lesson LOG School: Grade Level: Teacher: Science Teaching Dates/Time: QuarterDocument3 pagesDaily Lesson LOG School: Grade Level: Teacher: Science Teaching Dates/Time: Quarterjamel mayor100% (1)
- 10th-PHYSICS-Study Material For Slow learners-EMDocument25 pages10th-PHYSICS-Study Material For Slow learners-EMsheikjilanbashaNo ratings yet
- 4th Science Vi q4 w1 d2Document16 pages4th Science Vi q4 w1 d2vera marie pascual100% (1)
- Genchem 2 Module 2 Q2Document24 pagesGenchem 2 Module 2 Q2Kezia LuatNo ratings yet
- Lesson Plan For Gas LawDocument7 pagesLesson Plan For Gas LawCherry CaspeNo ratings yet
- SDLP-Kinetic Molecular TheoryDocument6 pagesSDLP-Kinetic Molecular TheoryJessica SudioNo ratings yet
- Pages 245-246 Pages-196-198: Grade 4Document7 pagesPages 245-246 Pages-196-198: Grade 4ALDRIN REYNOSONo ratings yet
- Dokumen PDF 23Document1 pageDokumen PDF 23Farhan FarhanNo ratings yet
- April Yadhi Xi.4Document2 pagesApril Yadhi Xi.4ordinarychansNo ratings yet
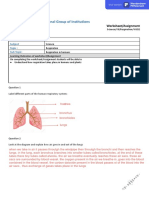
- Respiration: The Big Idea: Explore Different Aspects of The Respiratory SystemDocument6 pagesRespiration: The Big Idea: Explore Different Aspects of The Respiratory SystemYerick MartinezNo ratings yet
- Princess Garcia - MODULE 5 LABORATORY ACTIVITY FOR PRESSURE NAD FLUIDDocument13 pagesPrincess Garcia - MODULE 5 LABORATORY ACTIVITY FOR PRESSURE NAD FLUIDPrincess GarciaNo ratings yet
- Gen Chem 2 Q2 Module 2 - RemovedDocument20 pagesGen Chem 2 Q2 Module 2 - RemovedorevillojhnNo ratings yet
- Lesson Plan For Co 2Document5 pagesLesson Plan For Co 2Mareche AnildesNo ratings yet
- Class:7 Subject: Science Topic: Winds Storms and Cyclones: Summary Sheet Pedagogical Approach: Student - LedDocument15 pagesClass:7 Subject: Science Topic: Winds Storms and Cyclones: Summary Sheet Pedagogical Approach: Student - LedManpreet Kaur100% (4)
- Stellar Work! G12 First LabDocument9 pagesStellar Work! G12 First LabrovshanmirzakhanliNo ratings yet
- JASMIN - LP15 With Attached Worksheets - Classify Materials As Substances or MixturesDocument9 pagesJASMIN - LP15 With Attached Worksheets - Classify Materials As Substances or MixturesAbigail JasminNo ratings yet
- Gas Lab With QuestionsDocument3 pagesGas Lab With Questionsallan oparaNo ratings yet
- 7LAB16 U09 RespirationDocument6 pages7LAB16 U09 Respirationas61217No ratings yet
- Topic: Liquids Grade Level: Grade 8 Time Allotment: 1 Hour Teacher: ContentDocument3 pagesTopic: Liquids Grade Level: Grade 8 Time Allotment: 1 Hour Teacher: ContentTevoj OinolebNo ratings yet
- Human BodyDocument24 pagesHuman Bodyapi-734248685No ratings yet
- DLL Region-5Document5 pagesDLL Region-5Queen GarciaNo ratings yet
- 2a Diffusion Dissolving (2017)Document1 page2a Diffusion Dissolving (2017)Karina LeungNo ratings yet
- Boyle's Law Lesson PlanDocument5 pagesBoyle's Law Lesson PlanDaryl FCNo ratings yet
- Science 3 CotDocument3 pagesScience 3 Cotaileen.banagNo ratings yet
- GRACE Sea Breeze Land BreezeDocument6 pagesGRACE Sea Breeze Land BreezeJell Vicor OpenaNo ratings yet
- Delp-Science 3-Q1-W4Document5 pagesDelp-Science 3-Q1-W4Gom BearNo ratings yet
- Observations and Explanations: Report For Experiment 3Document3 pagesObservations and Explanations: Report For Experiment 3vmiznerNo ratings yet
- GraspsDocument3 pagesGraspsAngelou GloriaNo ratings yet
- DLL - SCIENCE 4 - Q3 - WEEK 5 Describe How Light, Sound and Heat TravelDocument8 pagesDLL - SCIENCE 4 - Q3 - WEEK 5 Describe How Light, Sound and Heat Travelkristine BurlaosNo ratings yet
- EXPERIMENT 1 Gen Chem With Org ChemDocument7 pagesEXPERIMENT 1 Gen Chem With Org ChemMariz del RosarioNo ratings yet
- Ryan International Group of Institutions: Class Subject Topic: Sub TopicDocument3 pagesRyan International Group of Institutions: Class Subject Topic: Sub Topicdaksh kumarNo ratings yet
- Curriculum Guide: Instructional PlanningDocument6 pagesCurriculum Guide: Instructional PlanningMERCEDITA S. TOJINONo ratings yet
- Smelly Balloon Diffusion Activity: Teacher DirectionsDocument4 pagesSmelly Balloon Diffusion Activity: Teacher DirectionsMariam KhalidNo ratings yet
- ENSC101L Manual 1.2BDocument8 pagesENSC101L Manual 1.2BMar IelleNo ratings yet
- Chapter 11 PDFDocument38 pagesChapter 11 PDFNick EsquidaNo ratings yet
- Lesson Plan Gagne Nin Step EventsDocument4 pagesLesson Plan Gagne Nin Step EventsSunil BajajNo ratings yet
- Dokumen PDF 38Document1 pageDokumen PDF 38Farhan FarhanNo ratings yet
- Dokumen PDF 42Document1 pageDokumen PDF 42Farhan FarhanNo ratings yet
- Dokumen PDF 36Document1 pageDokumen PDF 36Farhan FarhanNo ratings yet
- Dokumen PDF 45Document1 pageDokumen PDF 45Farhan FarhanNo ratings yet
- Dokumen PDF 32Document1 pageDokumen PDF 32Farhan FarhanNo ratings yet
- Dokumen PDF 46Document1 pageDokumen PDF 46Farhan FarhanNo ratings yet
- Dokumen PDF 28Document1 pageDokumen PDF 28Farhan FarhanNo ratings yet
- Dokumen PDF 58Document1 pageDokumen PDF 58Farhan FarhanNo ratings yet
- Dokumen PDF 26Document1 pageDokumen PDF 26Farhan FarhanNo ratings yet
- Dokumen PDF 9Document1 pageDokumen PDF 9Farhan FarhanNo ratings yet
- Dokumen PDF 12Document1 pageDokumen PDF 12Farhan FarhanNo ratings yet
- Dokumen PDF 2Document1 pageDokumen PDF 2Farhan FarhanNo ratings yet
- Dokumen PDF 4Document1 pageDokumen PDF 4Farhan FarhanNo ratings yet
- Review Material For Exam #3Document3 pagesReview Material For Exam #3quimicosorioNo ratings yet
- The Arrhenius Equation For Reversible ReactionsDocument4 pagesThe Arrhenius Equation For Reversible ReactionsmuratNo ratings yet
- Blackbody RadiationDocument9 pagesBlackbody RadiationTitin Evania ManaluNo ratings yet
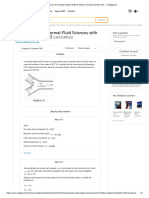
- Finite Element Method Sample Problem: 207 - 207 0 U F - 207 414 - 207 K U F 0 - 207 207 U FDocument2 pagesFinite Element Method Sample Problem: 207 - 207 0 U F - 207 414 - 207 K U F 0 - 207 207 U FJNAX GamingNo ratings yet
- Introduce To Probabilistic Machine LearningDocument53 pagesIntroduce To Probabilistic Machine LearningPin WangNo ratings yet
- SCR 2n5061Document8 pagesSCR 2n5061Gary NugasNo ratings yet
- Syllabus: PrimaryDocument45 pagesSyllabus: PrimaryTommy TingiNo ratings yet
- Class VII Science Chapter Heat WorksheetDocument2 pagesClass VII Science Chapter Heat Worksheetchandramani.goswamiNo ratings yet
- 2016 - Gershuny, Walter, Washburn - Effects of Viscosity On Bead Shape of Polydimethylsiloxane Fluid Flowing Down A Fiber - UnknownDocument9 pages2016 - Gershuny, Walter, Washburn - Effects of Viscosity On Bead Shape of Polydimethylsiloxane Fluid Flowing Down A Fiber - UnknownClaudio BiaginiNo ratings yet
- Relative Velocity: Interception and Collision by Ronald DdunguDocument17 pagesRelative Velocity: Interception and Collision by Ronald DdunguSam kellyNo ratings yet
- Analysis of The Track Sections Control System A Rolling Stock Axle Counting SensorDocument8 pagesAnalysis of The Track Sections Control System A Rolling Stock Axle Counting Sensorrizky maulidiyahNo ratings yet
- Denavit Hartenberg ParametersDocument21 pagesDenavit Hartenberg Parameters1DS19ME136-Shivam KumarNo ratings yet
- 06 - Rubbing Detection in A Synthesis Gas CompressorDocument10 pages06 - Rubbing Detection in A Synthesis Gas CompressorSeresdfrtNo ratings yet
- VIV Tandem Diamond IJMS 2023Document49 pagesVIV Tandem Diamond IJMS 2023Intesaaf AshrafNo ratings yet
- Obtaining Cellulose Nanofibers With A Uniform Width of 15 NM From WoodDocument3 pagesObtaining Cellulose Nanofibers With A Uniform Width of 15 NM From Wooddhy182No ratings yet
- Kannur University Appointment Notification Dated 15.06.2022 1Document3 pagesKannur University Appointment Notification Dated 15.06.2022 1Joseph I ThomasNo ratings yet
- Lecture 1 (Introduction)Document18 pagesLecture 1 (Introduction)mNo ratings yet
- Linear RegressionDocument7 pagesLinear Regressionmatthew chombaNo ratings yet
- Yearly Teaching Plan Summary Maths f2 KSSMDocument2 pagesYearly Teaching Plan Summary Maths f2 KSSMliza68No ratings yet
- Solved A Hot Water Stream at 80°C Enters A Mixing Chamber With CheggDocument3 pagesSolved A Hot Water Stream at 80°C Enters A Mixing Chamber With Cheggsalim.22en1No ratings yet
- Centrifuge SIGMA 3-16K Operating ManualDocument69 pagesCentrifuge SIGMA 3-16K Operating ManualVitor FilipeNo ratings yet
- Sfa Vessels Guide July22Document40 pagesSfa Vessels Guide July22Ilias ZilakosNo ratings yet
- Ikaapat-na-Markahang-Pagsusulit-sa-MATHEMATICS-IVDocument5 pagesIkaapat-na-Markahang-Pagsusulit-sa-MATHEMATICS-IVaiselpesanosNo ratings yet
- Calculus Session 1 Area of A Region in A PlaneDocument4 pagesCalculus Session 1 Area of A Region in A PlaneJennie Gail RigorNo ratings yet
- Delayline Probes - SonatestDocument2 pagesDelayline Probes - SonatestRajaprasannakumarDPillaiNo ratings yet
- EN3: Introduction To Engineering and Statics: 3. Resultant of Systems of ForcesDocument6 pagesEN3: Introduction To Engineering and Statics: 3. Resultant of Systems of ForceskarthikaNo ratings yet