Professional Documents
Culture Documents
Review Midtest Grade 7
Uploaded by
Putra Habib DhitarekaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Review Midtest Grade 7
Uploaded by
Putra Habib DhitarekaCopyright:
Available Formats
Review Midtest
Grade 7
A. Chapter 5 States of Matter
Show in outline how the particle theory of matter can be used to explain the properties of
solids, liquids and gases, including changes of state
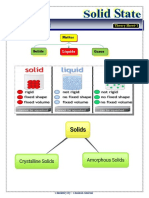
State of matter
Solid Liquids Gases
Keep the same volume Keep the same volume Can change the volume
Take the same amount of Take the same amount of Can change the amount of
space space shape
Keep the same shape Take the shape of the Take the shape of closed
The particle are arranged in container container
fix pattern because the The particle can move past The particle do not touch
attractive force between one another because the each other because the
the particle is really strong attractive force between attractive force between
particle is enough to make the particle is weak
them stay together
Change of matter
Sublimation Gas Evaporation Gas Condensation Liquid
Solid Liquid Gas n
Liquid Solid Solid
Melting Freezing Deposition
d
B. Chapter 6 Material Properties
1. Distinguish between metals and non-metals
Metal Some metals are magnetic
Most are solid in room Non-Metal
temperature Many are gases at room
They are shinny temperature
They do not shatter They are dull
They conduct heat energy well They are brittle
They conduct electricity They do not conduct heat energy
They are melleable well
They are ductile Most do not conduct electricity
They make a ringiing sound when Solid and Liquid non-metal have
hit low melting points and boiling
Most of metal have high boiling points
and melting point Do not reflect light very well
2. Describe everyday materials and their physical properties
Plastic
Some plastic are flexible, Ceramics
lightweight, and easiy shaped Withstand high temperature
Plastic do not react with the food Hard, brittle, very strong
Plastic takes a very long time to
break down Glass
Transparent or transculent
Fibres Hard but very brittle
Tinny threads Waterproof and do not react with
Strong and bendy food
Made from natural or synthetic
C. Chapter 7 Material Changes
1. Use a pH scale
Acid : pH 0 6,9
Neutral : pH 7
Alkaline : pH 7,1 14
2. Understand neutralisation and some of its applications
Neutralisation process occur when you mix acid and alkaline together. They will
produce salt and water which are neutral.
The apllication of neutralisation process in daily life:
1. Acid in our stomach and alkaline solution from indigestion medicine
2. Acid from bacteria in our mouth and alkaline from toothpaste
3. Use indicators to distinguish acid and alkaline solutions
Indicator is any substance that gives visible sign to show how acidic or alkaline a
substane is.
Litmus paper is the example of indicator. It will turn red when it is put in acid
and turn blue when it is put in alkaline.
The good indicator is the one that can distinguish acid, neutral and alkaline
The example of natural indicator are red cabbage, turmeric, flower petals etc.
You might also like
- GCSE Chemistry Revision: Cheeky Revision ShortcutsFrom EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsRating: 4.5 out of 5 stars4.5/5 (3)
- Swiss Army Triplet 1Document2 pagesSwiss Army Triplet 1johnpwayNo ratings yet
- Dragons ScaleDocument13 pagesDragons ScaleGuilherme De FariasNo ratings yet
- Matter and ITS Properties: Arvin C. Diamante Master Teacher II Nicolas L. Galvez Memorial NHS June 18, 2018Document57 pagesMatter and ITS Properties: Arvin C. Diamante Master Teacher II Nicolas L. Galvez Memorial NHS June 18, 2018Arvin Corpuz DiamanteNo ratings yet
- Chemistry 10 - 12Document333 pagesChemistry 10 - 12Théé Néw SåmûNo ratings yet
- IGCSE Chemistry S1 L1Document116 pagesIGCSE Chemistry S1 L1Antonia Putri Sri Nova YolandhaNo ratings yet
- Children Encyclopedia Chemistry: The World of KnowledgeFrom EverandChildren Encyclopedia Chemistry: The World of KnowledgeRating: 5 out of 5 stars5/5 (3)
- Chap 5 MatterDocument32 pagesChap 5 MatterAimi Nadia Yusof71% (7)
- Academic Performance of Senior High School Students 4Ps Beneficiaries in VNHSDocument19 pagesAcademic Performance of Senior High School Students 4Ps Beneficiaries in VNHSkathlen mae marollanoNo ratings yet
- MATTERDocument49 pagesMATTERHakdog100% (1)
- Amerex Ansul Badger Ul Catalogo Por PartesDocument37 pagesAmerex Ansul Badger Ul Catalogo Por PartesPuma De La Torre ExtintoresNo ratings yet
- A Detailed Lesson PlanDocument5 pagesA Detailed Lesson PlanIsaac-elmar Agtarap74% (23)
- 1.nature of MatterDocument31 pages1.nature of MatterKate IgtosNo ratings yet
- Or HandoutDocument190 pagesOr Handoutyared haftu67% (6)
- Gen Chem Chapt.1Document45 pagesGen Chem Chapt.1Dave Cercado BugadorNo ratings yet
- Final LUS EvaluationDocument36 pagesFinal LUS EvaluationNextgenNo ratings yet
- 03 IGT-Influence of Codes Guidelines and Other Regulations On The Tunnel Design in AustriaDocument48 pages03 IGT-Influence of Codes Guidelines and Other Regulations On The Tunnel Design in AustriaSudarshan GadalkarNo ratings yet
- AIP 2020 FINAL JuneDocument5 pagesAIP 2020 FINAL JuneVINA ARIETANo ratings yet
- Reviewer in Science 8 3RD QuarterDocument5 pagesReviewer in Science 8 3RD QuarterJillianNo ratings yet
- Presented by Nermin Mohamed Sabry: States of MatterDocument6 pagesPresented by Nermin Mohamed Sabry: States of Matterelagamy_2000No ratings yet
- Matter HandoutDocument2 pagesMatter Handoutgigizamoras47No ratings yet
- Solid State - PLPN MhtCetDocument42 pagesSolid State - PLPN MhtCetsiddheshmundlik6No ratings yet
- MAT T Er in Our SurroundingDocument8 pagesMAT T Er in Our SurroundingBharathNo ratings yet
- Guide February IP 2019 Natural Sciences and Environmental Education - Biology and Chemistry Seventh GradeDocument3 pagesGuide February IP 2019 Natural Sciences and Environmental Education - Biology and Chemistry Seventh GradeLucy GarcesNo ratings yet
- College Notes Unit-1 Solid StateDocument24 pagesCollege Notes Unit-1 Solid StateRamanujam JNo ratings yet
- Chapter 5 Matter in NatureDocument1 pageChapter 5 Matter in NatureYuhannNo ratings yet
- Lesson 1: Matter and Its PropertiesDocument13 pagesLesson 1: Matter and Its Propertiesricky100% (1)
- The Particulate NatureDocument123 pagesThe Particulate Naturewidya sariNo ratings yet
- IGCSE Chemistry A - Notes Chapter 1 - The Particulate Nature of MaterDocument25 pagesIGCSE Chemistry A - Notes Chapter 1 - The Particulate Nature of MaterDhingra shellyNo ratings yet
- MatterDocument40 pagesMatterMarianne B. HingpesNo ratings yet
- Inorganic Chemistry: Bachelor of Science in Pharmacy Alyn Tapleras, MSPH - RPHDocument26 pagesInorganic Chemistry: Bachelor of Science in Pharmacy Alyn Tapleras, MSPH - RPHNur-aine HajijulNo ratings yet
- Materials and Their Properties 1Document3 pagesMaterials and Their Properties 1MARIANA SERNA LOPEZNo ratings yet
- Example: Sun or Any StarDocument3 pagesExample: Sun or Any StarNiki KevinNo ratings yet
- Gases vs. Liquids and SolidsDocument67 pagesGases vs. Liquids and SolidsRenolsa EspinosaNo ratings yet
- Chap 1 Particulate Nature of MatterDocument71 pagesChap 1 Particulate Nature of MatterAqua StarNo ratings yet
- Revision - Properties of MaterialsDocument7 pagesRevision - Properties of MaterialshuongttNo ratings yet
- ODB - Chem (Matter)Document2 pagesODB - Chem (Matter)aloevera1994100% (1)
- CHAPTER 1 Matter, MeasurementsDocument23 pagesCHAPTER 1 Matter, MeasurementsRusher SigueNo ratings yet
- Gen Chem 2 Module 1 AnswersDocument6 pagesGen Chem 2 Module 1 AnswersSharmaine Joyce RegioNo ratings yet
- Matter 65Document13 pagesMatter 65Venkateswar PatroNo ratings yet
- Chemistry 10 - 12 PDFDocument283 pagesChemistry 10 - 12 PDFRon ShamendeNo ratings yet
- 1GP - Chemistry NotesDocument12 pages1GP - Chemistry NoteseriannenabazengNo ratings yet
- Objectives:: Key Questions and Terms NotesDocument2 pagesObjectives:: Key Questions and Terms NotesfelishaNo ratings yet
- Materials and ThingsDocument2 pagesMaterials and Thingsamritha mishraNo ratings yet
- Chapter 1 MatterDocument79 pagesChapter 1 MatterdwyquishNo ratings yet
- Chemistry Checkpoint Topics Part 1Document28 pagesChemistry Checkpoint Topics Part 1esteban ferrada silvaNo ratings yet
- Diversity of Materials in The EnvironmentDocument19 pagesDiversity of Materials in The EnvironmentMarjorie TrinidadNo ratings yet
- Gen Chem NatennnDocument5 pagesGen Chem NatennnAriaane Grace DaquioagNo ratings yet
- World of MatterDocument14 pagesWorld of MatterAding SamNo ratings yet
- Structure and Properties of Water: General Chemistry 2Document38 pagesStructure and Properties of Water: General Chemistry 2Cielo GatdulaNo ratings yet
- SLIDE 1 - Sci 07 (Matter)Document81 pagesSLIDE 1 - Sci 07 (Matter)Allen Joy LazoNo ratings yet
- T SC 2550160 Ks2 Year 5 Properties and Changes of Materials Revision Activity Mat Ver 4Document6 pagesT SC 2550160 Ks2 Year 5 Properties and Changes of Materials Revision Activity Mat Ver 4khushbakhtNo ratings yet
- Chemistry: Class - 12 SubjectDocument7 pagesChemistry: Class - 12 SubjectHemant YadavNo ratings yet
- Chem HandoutDocument11 pagesChem HandoutEllen Mae PrincipeNo ratings yet
- General Chemistry 2: Quarter 1-Module 3Document31 pagesGeneral Chemistry 2: Quarter 1-Module 3Niño Edrianne NimoNo ratings yet
- Sci P.5.5A Properties of Matter NotesDocument3 pagesSci P.5.5A Properties of Matter Notesnjuhatlast16No ratings yet
- 4.sorting Out Materials and Objects-NotesDocument3 pages4.sorting Out Materials and Objects-NotesmohaddisaNo ratings yet
- Unit 9 Particle Nature of MatterDocument67 pagesUnit 9 Particle Nature of Mattermiguelcastillo212301No ratings yet
- Exercise 1 - Matter and Its PropertiesDocument3 pagesExercise 1 - Matter and Its PropertiespututuPLNo ratings yet
- Ram Gelo de Luna Haway - ACTIVITY NO. 1 (General Chemistry)Document5 pagesRam Gelo de Luna Haway - ACTIVITY NO. 1 (General Chemistry)Ram Gelo HawayNo ratings yet
- St. Paul School of Aparri: Matter (Solid, Lquid, and Gas)Document21 pagesSt. Paul School of Aparri: Matter (Solid, Lquid, and Gas)Alzen GalaponNo ratings yet
- MATTERDocument49 pagesMATTERHakdogNo ratings yet
- Module 1 Properties and Structure of MatterDocument17 pagesModule 1 Properties and Structure of Matterisaheqq12No ratings yet
- C1 - Notes 1 of 2 - Solid StateDocument5 pagesC1 - Notes 1 of 2 - Solid StateAtharva BhavsarNo ratings yet
- CHAPTER - MATTER (Combined PPT) Class VII Help NotesDocument72 pagesCHAPTER - MATTER (Combined PPT) Class VII Help NotesPriyanca JunejaNo ratings yet
- Genchem ReviewerDocument3 pagesGenchem ReviewerKarylle PingolNo ratings yet
- Intro To Inorg ChemDocument40 pagesIntro To Inorg Chemelizabethabraham310No ratings yet
- Gen Chem - Week 2Document4 pagesGen Chem - Week 2Faith Maiden MusaNo ratings yet
- 1 Principles of Chemistry 19Document196 pages1 Principles of Chemistry 19LaziNo ratings yet
- A Homey School For Future Leaders: Pelita Nusantara School National & International CurriculumDocument2 pagesA Homey School For Future Leaders: Pelita Nusantara School National & International CurriculumPutra Habib DhitarekaNo ratings yet
- Lesson Plan 7A-1 (7Cc1 7Cc2) RevisiDocument2 pagesLesson Plan 7A-1 (7Cc1 7Cc2) RevisiPutra Habib DhitarekaNo ratings yet
- Lesson Plan 7A-3 (7ce1 7ce2)Document2 pagesLesson Plan 7A-3 (7ce1 7ce2)Putra Habib DhitarekaNo ratings yet
- Lesson Plan 7A-3 (7ce1 7ce2)Document2 pagesLesson Plan 7A-3 (7ce1 7ce2)Putra Habib DhitarekaNo ratings yet
- Lesson Plan 7A-5 (7Pf1 7Pf2)Document2 pagesLesson Plan 7A-5 (7Pf1 7Pf2)Putra Habib DhitarekaNo ratings yet
- Putra Habib D-1304681-Comparative ArticleDocument1 pagePutra Habib D-1304681-Comparative ArticlePutra Habib DhitarekaNo ratings yet
- Putra Habib D-1304681-Book Report - Biografi Umar Bin Al-KhathabDocument3 pagesPutra Habib D-1304681-Book Report - Biografi Umar Bin Al-KhathabPutra Habib DhitarekaNo ratings yet
- Planets TableDocument4 pagesPlanets TablePutra Habib DhitarekaNo ratings yet
- Putra Habib D-1304681-Presentation Report - Ethic On Using SmartphoneDocument3 pagesPutra Habib D-1304681-Presentation Report - Ethic On Using SmartphonePutra Habib DhitarekaNo ratings yet
- Putra Habib D-1304681 - Seminar Paper - Seminar On IPTEKSEN - Ethic On Using SmartphoneDocument38 pagesPutra Habib D-1304681 - Seminar Paper - Seminar On IPTEKSEN - Ethic On Using SmartphonePutra Habib DhitarekaNo ratings yet
- Fun With Simple Machines Fun With Simple MachinesDocument2 pagesFun With Simple Machines Fun With Simple MachinesPutra Habib DhitarekaNo ratings yet
- S M - T o W NDocument2 pagesS M - T o W NPutra Habib DhitarekaNo ratings yet
- Putra Habib D-1304681 - Paper Journal - Smartphone Usage in Islamic PerspectivesDocument11 pagesPutra Habib D-1304681 - Paper Journal - Smartphone Usage in Islamic PerspectivesPutra Habib DhitarekaNo ratings yet
- The Planets: Solar SystemDocument4 pagesThe Planets: Solar SystemPutra Habib DhitarekaNo ratings yet
- Nada 3 (Habib) : NeptuneDocument2 pagesNada 3 (Habib) : NeptunePutra Habib DhitarekaNo ratings yet
- Bagan Diagram ImmersedDocument1 pageBagan Diagram ImmersedPutra Habib DhitarekaNo ratings yet
- C&C - Putra Habib and Galuh Ayuningtyas - 1304681 - Pribadi SchoolDocument2 pagesC&C - Putra Habib and Galuh Ayuningtyas - 1304681 - Pribadi SchoolPutra Habib DhitarekaNo ratings yet
- Lesson Plan: Immersed ModelDocument4 pagesLesson Plan: Immersed ModelPutra Habib DhitarekaNo ratings yet
- Educational Leadership A. The Meaning of Educational LeadershipDocument5 pagesEducational Leadership A. The Meaning of Educational LeadershipPutra Habib DhitarekaNo ratings yet
- ReferenceDocument1 pageReferencePutra Habib DhitarekaNo ratings yet
- Putra H.D 1304681 (Construct-Simple Machines)Document4 pagesPutra H.D 1304681 (Construct-Simple Machines)Putra Habib DhitarekaNo ratings yet
- Learning MaterialDocument28 pagesLearning MaterialPutra Habib DhitarekaNo ratings yet
- Scientific Theories and ProgressDocument29 pagesScientific Theories and ProgressPutra Habib DhitarekaNo ratings yet
- Assessment INT SCIDocument5 pagesAssessment INT SCIPutra Habib DhitarekaNo ratings yet
- Science and Humankind: Cladinea Mahfira (1304593) R. Auliya U.I.F (1304313)Document25 pagesScience and Humankind: Cladinea Mahfira (1304593) R. Auliya U.I.F (1304313)Putra Habib DhitarekaNo ratings yet
- Relativism, Instrumentalism, and RelevanceDocument21 pagesRelativism, Instrumentalism, and RelevancePutra Habib DhitarekaNo ratings yet
- Concept Analysis of Energy Topic: Submitted As One Assignment of School Science 2Document10 pagesConcept Analysis of Energy Topic: Submitted As One Assignment of School Science 2Putra Habib DhitarekaNo ratings yet
- Predictability and DeterminismDocument34 pagesPredictability and DeterminismPutra Habib DhitarekaNo ratings yet
- Realism, Language, and ReductionismDocument29 pagesRealism, Language, and ReductionismPutra Habib DhitarekaNo ratings yet
- Concept Analysis (Electricity)Document11 pagesConcept Analysis (Electricity)Putra Habib DhitarekaNo ratings yet
- God Reproducing Himself in UsDocument6 pagesGod Reproducing Himself in UsLisa100% (1)
- Handout Waste Catch BasinDocument2 pagesHandout Waste Catch BasinJonniel De GuzmanNo ratings yet
- Final Quiz 2 - Attempt ReviewDocument6 pagesFinal Quiz 2 - Attempt Reviewkoraijohnson7No ratings yet
- GR 9 Eng CodebDocument6 pagesGR 9 Eng CodebSharmista WalterNo ratings yet
- ASTR 323 Homework 4Document2 pagesASTR 323 Homework 4Andrew IvanovNo ratings yet
- Pot-Roasted Beef BrisketDocument4 pagesPot-Roasted Beef Brisketmarcelo nubileNo ratings yet
- A-1660 11TH Trimester From Mcdowell To Vodafone Interpretation of Tax Law in Cases. OriginalDocument18 pagesA-1660 11TH Trimester From Mcdowell To Vodafone Interpretation of Tax Law in Cases. OriginalPrasun TiwariNo ratings yet
- Sistemas de Mando CST Cat (Ing)Document12 pagesSistemas de Mando CST Cat (Ing)Carlos Alfredo LauraNo ratings yet
- Questions 1 To 3 Are Based On The Following TextDocument7 pagesQuestions 1 To 3 Are Based On The Following TextHokage KumNo ratings yet
- Speaking RubricDocument1 pageSpeaking RubricxespejoNo ratings yet
- RESEARCHDocument5 pagesRESEARCHroseve cabalunaNo ratings yet
- Oral Communication in ContextDocument19 pagesOral Communication in ContextAzory ZelleNo ratings yet
- CESCOM 10 - Aircraft Status Report: Maintenance InspectionsDocument78 pagesCESCOM 10 - Aircraft Status Report: Maintenance InspectionsAlejandro BarradasNo ratings yet
- Week 3 Alds 2202Document13 pagesWeek 3 Alds 2202lauren michaelsNo ratings yet
- M.SC Food NutritionDocument44 pagesM.SC Food Nutritionasu reddyNo ratings yet
- DIY Toolkit Arabic Web VersionDocument168 pagesDIY Toolkit Arabic Web VersionAyda AlshamsiNo ratings yet
- 00022443the Application of A Continuous Leak Detection System To Pipelines and Associated EquipmentDocument4 pages00022443the Application of A Continuous Leak Detection System To Pipelines and Associated EquipmentFaizal AbdullahNo ratings yet
- Bullying Report - Ending The Torment: Tackling Bullying From The Schoolyard To CyberspaceDocument174 pagesBullying Report - Ending The Torment: Tackling Bullying From The Schoolyard To CyberspaceAlexandre AndréNo ratings yet
- Aex-Kissan KeralaDocument25 pagesAex-Kissan Keralabsh08070No ratings yet
- Dress Code19sepDocument36 pagesDress Code19sepapi-100323454No ratings yet
- Gigabyte Ga b85m Ds3h A r10 PDFDocument30 pagesGigabyte Ga b85m Ds3h A r10 PDFMartha Lorena TijerinoNo ratings yet