Professional Documents
Culture Documents
11 Mechanical Properties of Fluids 2023-24.
Uploaded by
parvezanas7Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
11 Mechanical Properties of Fluids 2023-24.
Uploaded by
parvezanas7Copyright:
Available Formats
CLASS – 11
PHYSICS
Mechanical Properties of Fluids
Contact – 9910868296; 9811017067
Mechanical Properties of Fluids Page 1 of 32
PROPERTIES OF STATIC FLUID
Fluid
A fluid is a substance that can flow. It ultimately assumes the shape of the containing
vessel because it cannot withstand shearing stress. Thus, both liquids and gases are
fluids.
Important characteristics of fluids:-
a) The atoms or molecules in a fluid are arranged in a random manner.
b) A fluid cannot withstand tangential or shearing stress for an indefinite period. It begins to
flow when a shearing stress is applied.
c) A fluid has no definite shape of its own. It ultimately assumes the shape of the containing
vessel. So a fluid has ho modulus of rigidity.
d) A fluid can exert/withstand a force in a direction perpendicular to its surface. So a fluid does
have a bulk modulus of rigidity.
Difference between liquid and gas
A liquid is incompressible and has a definite volume and a free surface of its own. A gas is
compressible and it expands to occupy all the space available to it.
Thrust of a liquid
When a liquid is in equilibrium, forces acting on the liquid must act perpendicular to its
surface.
It can be easily proved as below:
Consider a liquid contained in a vessel. Suppose that the liquid is in equilibrium state of rest
and a force F is acting along OA makes an angle θ with the horizontal as shown in figure.
The force F can be resolved into two components:
(i) F cos θ along horizontal and
(ii) F sinθ along vertical.
Since the liquid is at rest (there is no flow of liquid), the
force along the horizontal must be zero i.e. F cos θ = 0
As F cannot be zero, we have
cos θ = 0 or θ = 90°
Hence, if a liquid is in equilibrium or state of rest, then force (or forces) acting on the
fluid must be perpendicular to its surface and called thrust.
Pressure
The thrust exerted by a liquid (at rest) per unit area of the surface in contact with the
liquid is called pressure. Pressure is a scalar quantity.
S. I. unit – Pascal or N/m2
Dimension − [ML−1 T −2]
Application of Pressure
(i) Paper-pins and nails are made to have pointed ends. Due to this, the pin if pressed, will
exert high pressure on the surface, and hence will easily penetrate the surface.
(ii) The bags and suitcases are provided with broad handles so that small pressure is exerted
on the hand while carrying them.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 2 of 32
(iii) It is painful to walk bare footed on a road covered with edge pebbles. It is due to the fact
that if our body weight is supported on a very small area of the sharp edge of the pebble on
the road, it will exert a large pressure on our feet.
(iv) Railway tracks are laid on large sized wooden/concrete sleepers so that the thrust due to
weight of train is spread over a large area. This reduces the pressure on ground which would
prevent the yielding of ground.
(v) One can walk on sand easily by placing wooden planks on the sand. In this situation, the
thrust on the sand due to our weight will get spread over the whole area of the board and
hence a very small pressure is exerted on the sand. Due to which, the sand does not yield.
PRESSURE EXERTED BY A LIQUID COLUMN
Consider a liquid contained in a cylindrical vessel of cross sectional area ‘a’. Let h be the
height of liquid column, ρ be its density and g be the acceleration due to gravity. The weight
of liquid will exert a downward thrust on the bottom surface of the vessel. Therefore,
pressure due to liquid acts on that surface.
Weight of liquid inside the vessel
= volume × density of liquid × acc. due to gravity
=ah×ρ×g
Thrust of liquid on area a = weight of liquid = a h ρ g
Thus, Liquid pressure on the base of vessel is
thrust ahρg
P= area
= a
P=h ρg
Some Facts –
1. The liquid at rest exerts equal pressure in all directions at a point Inside the liquid.
2. The liquid at rest exerts equal pressure at all those points which are at one level inside the
liquid.
3. Liquid pressure is independent of shape of the liquid surface as well as the area of the liquid
surface, but depends upon the height at liquid column.
4. Total pressure at a depth h below the liquid surface = P0 + h ρ g; where P0 = atmospheric
pressure.
5. Mean pressure on the walls of a beaker containing liquid upto height h is (= hρg/2), where ρ
is the density of liquid.
6. Thrust exerted by liquid on the walls of the vessel in contact with liquid is normal to the
surface of vessel.
Variation of liquid pressure with depth
As shown in Figure consider a liquid at rest in a container. The liquid pressure must be same
at all points which are at the same depth, as otherwise liquid will not be in equilibrium.
Imagine a cylindrical element of the liquid of cross-sectional area A and height h. Let P1 and
P2 be the liquid pressures at its top point 1 and bottom point 2 respectively.
As the liquid cylinder is at rest, the resultant horizontal force should be zero. Various acting
on it in the vertical direction are:
(i) Force due to the liquid pressure at the top,
F1 = P1 A, acting downwards
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 3 of 32
(ii) Force due to the liquid pressure at the bottom,
F2 = P2 A, acting upwards
(iii) Weight of the liquid cylinder acting downwards,
W = Mass x g = Volume x density x g
= Ahρg
where 𝜌 is the density of the liquid.
As the liquid cylinder is in equilibrium,
Net downward force =Net upward force or
F1 + W = F2
Or F2 - F1 = W
or P2A- P1A = Ahρg
or P2 - P1 = hρg
If we shift point 1 to the liquid surface, which is open to the atmosphere, then we can replace
P1 by atmospheric pressure Pa and P2 by P in the above equation and we get
P – Pa = h𝜌g
P = Pa + h𝜌g
We can note the following points:
1) The liquid pressure is the same at all points at the same horizontal level or at same depth.
2) Pressure at any point inside the fluid depends on the depth h.
3) The absolute (actual) pressure P, at a depth h below the liquid surface open to the atmosphere
is greater than the atmospheric pressure by an amount hpg. The excess pressure P - Pa, at
depth h is called a gauge pressure at that point.
4) Pressure does not depend on the cross-section or base-area or the shape of the vessel.
PASCAL’S LAW
Pascal’s law states that “in an enclosed fluid, if an increased pressure is produced in
any part of the fluid, then this change of pressure is transmitted undiminished to all
the other parts of the fluid and also to the walls of the container, provided the effect of
gravity is neglected”.
Experimental verification of pascal’s law
1. Take a hollow rubber ball and make a number of fine pin holes at different places over the
surface of the ball. Pour water into the ball through a wider hole. After closing the wider
hole with a finger; if we squeeze the ball, water will be seen to rush out of the pin holes in
the shape of fine streams. Further, the streams of water will reach up to the same distance in
air indicating that the increased pressure on the water has been transmitted equally in all
directions.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 4 of 32
2. Take a vessel having three side tubes A, B and C and provided with frictionless and water
tight pistons. Suppose that the cross-sectional areas of the pistons A, B and C are ‘a’, ‘2a’ and
‘a/2’ respectively [see figure].
If thrust F is applied en the piston of the side tube A, then so that the pistons of the side
tubes B and C do not move, thrust equal to 2F and F/2 respectively will have to be applied
on them. It indicates that the pressure (and not thrust) is transmitted equally in all the
F
directions, which in the three cases is equal to a .
Effect of gravity on Pascal's law
If we neglect the effect of gravity, then
P2 - Pl = hpg = 0
That is, pressure at all points inside the liquid is same in the absence of gravity. This is
Pascal's law.
However, in the presence of gravity, Pascal's law gets modified as
P2 - P1 = h𝜌g
HYDRAULIC LIFT
A hydraulic lift is an arrangement used to multiply the force.
Principle – Hydraulic lift is based on the principle of Pascal's law. When a small force is
applied over a piston of small cross-sectional area, then due to transmission of pressure
equally in all directions, a large force appears over a piston of large cross-sectional area,
which is then utilised to support and lift heavy weights.
Construction:-
Basically, a hydraulic lift consists of two cylinders C and C' connected to each other with a
pipe. The two cylinders are of different areas of cross-sections and are provided with
frictionless pistons as shown in figure. Let ‘a’ and ‘A’ be the cross-sections of the pistons in
cylinders C and C', where a << A. The cylinders are filled with an incompressible fluid.
Working:-
Suppose that a small downward force f is applied on the smaller piston of cross-sectional area
‘a’. Then, pressure exerted over the liquid,
f
P=a
According to Pascal's law, the same pressure is transmitted to the larger piston of cross-
sectional area A. Then, force transmitted to larger piston C' is given by
f A
F=PA= a
A = f a
Since ‘A’ >> ‘a’, therefore force F >> f
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 5 of 32
This shows that a small force applied on the smaller piston results as a very large force on the
larger piston. As a result of which a heavy load placed on the larger piston is then easily
lifted.
HYDRAULIC BRAKES
The principle of the hydraulic brake used in vehic1es is same as that of the hydraulic lift i.e.
based on Pascal’s Law.
Construction –
It consists of a master cylinder M filled with brake oil and provided with air tight frictionless
piston P. The piston is connected to brake pedal F through lever system L. The master
cylinder is connected to wheel cylinder C through a tube T. The wheel cylinder is having two
pistons P1 and P2. These pistons are connected to brake shoes S1 and S2 respectively. The
spring S holds S1 and S2 in position. The similar system is connected to all the wheels of a
vehicle.
Working –
When the driver of the vehicle puts pressure on the brake paddler the lever system moves a
piston into the master cylinder containing brake fluid. The brake fluid from the master
cylinder is led through strong pipes to cylinders provided with pistons (P1, P2) of larger cross-
sectional area for brakes for different wheels. Thus, a small force applied over the brake
paddle is transmitted by the brake fluid as a large force to the piston for each brake. Due to
this, the brake shoes open up wider. They press against the brake linings of the drums of the
wheels and bring them to rest i.e. the brake become operative.
When the paddle is released, a spring system brings the brake shoes back to their normal
positions and the brake fluid returns to the master cylinder.
HYDROSTATIC PARADOX
Pascal demonstrated experimentally that the pressure exerted by a liquid column depends
only on the height of the liquid column and not on the shape of the containing vessel. As
shown in Fig. use, the experimental arrangement consists of three glass vessels A, B and C of
different shapes. The area of the lower open end of all the vessels is same. The lower end of
each vessel is closed by supporting a disc against it. Each disc is connected to a pressure-
meter.
When the three vessels are filled with the same liquid upto the same height, all the three
meters record the same pressure. This appears anomalous because the three vessels have
different shapes and contain different amounts of liquid. This apparently unexpected result is
known as hydrostatic paradox.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 6 of 32
ATMOSPHERIC PRESSURE
The atmospheric pressure at any point is equal to the weight of a vertical column of air
of unit cross-sectional area extending from that point-to the top of the earth's
atmosphere.
The atmospheric pressure is maximum at the surface of earth and goes on decreasing as we
move up into the earth's atmosphere. The value of atmospheric pressure on the surface of
earth at sea level called one atmosphere (1 atm.) is nearly 1.013 × l05 N/m2 or pascal in S.I,
Experiment to show the existence of atmospheric pressure –
1. Take a metal tin with one opening to which a vacuum pump is connected. Evacuate the air or
gas filled inside the tin. We shall note that the tin crumbles in shape by the force of
atmospheric pressure acting on its outer side.
2. Take a glass tumbler. Fill it with water up to brim. Slide carefully a cardboard on its rim so
that no air remains in between cardboard and water in tumbler. Invert the tumbler gently. We
shall see that water does not fall down. This is due to an upward thrust acting on the lower
surface of the cardboard by virtue of atmospheric pressure, which is balancing the weight of
water in the tumbler.
TORRICELLI'S EXPERIMENT
Torricelli was the first to devise an experiment for measuring atmospheric pressure.
Take a graduated hard glass tube 1m in length and of
uniform area of cross-section, closed at one end. Fill
the whole tube with pure and dry mercury taking care
that no air/water droplet remains inside the tube. Close
the open end of tube tightly with your thumb and invert
the tube. Take this inverted end of the tube along with
your thumb inside the pure and dry mercury contained
in a trough. Remove the thumb, taking care that the end
of the tube remains inside the mercury trough.
Hold the tube vertically as shown in figure. We find
that the mercury in the tube will fall down at first and
then will be stopped at a particular position.
The height of level of mercury in the tube is nearly 76 cm above the free level of mercury in
the trough. If the given tube is inclined or raised up or lowered in mercury trough, the vertical
height of mercury level in tube is always found to be constant.
Torricelli explained this by saying that the mercury column is supported by the atmospheric
pressure acting on the free surface of mercury in the trough. Hence the hydrostatic pressure
exerted by the vertical mercury column in the tube above the free surface of mercury in
the trough measures the atmospheric pressure. The empty space above mercury level in
the tube has a perfect vacuum, which is called Torricellian vacuum.
At sea level, atmospheric pressure is the pressure exerted by 0.76 m of mercury column.
The density of mercury, ρ = 13.6 × 103 kg/m3 and g = 9·8 ms-2
Atmospheric pressure, P = hρg
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 7 of 32
= 0·76 × 13·6 × 103 × 9·8
= 1.013 × 105 N/m2 or Pa
Note:- Pressure due to vertical column dose not depend on the inclination of the tube.
Height of Atmosphere –
Atmosphere of the earth exerts a pressure of 1.013 × 105 N/m2 on the surface of earth.
Consider the atmospheric air to be of uniform density = 1.3 kg/m3.
Then height of the atmosphere will be given by
F 1.013 × 105
h = ρg = = 7951 m = 8 km
1.3 ×9.8
Open-tube manometer
It is a simple device used to measure the pressure of a gas enclosed in a vessel. It consists of a
U-tube containing some liquid. One end of the tube is open to the atmosphere and the other
end is connected to the vessel. The total pressure P of the gas is equal to the pressure at A.
Thus, P = PA = Pc + h 𝜌 g or P = Pa + h 𝜌 g
where P is the atmospheric pressure,
h = BC = difference in the levels of the liquid
in the two arms and
𝜌 is the density of the liquid.
Absolute pressure and gauge pressure
The total or actual pressure P at a point is called absolute pressure. Gauge pressure is the
difference between the actual pressure (or absolute pressure) at a point and the atmospheric
pressure, i.e.,
Pg = P - Patm = h ρ g
The gauge pressure is proportional to h. Many pressure measuring devices directly measure
the gauge pressure. These include the tyre pressure gauge and the blood pressure gauge
(sphygmomanometer).
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 8 of 32
DYANAMIC FLUID
VISCOSITY
Viscosity is the property of fluid by virtue of which an internal force of friction comes
into play when a fluid is in motion and which opposes the relative motion between its
different layers. The backward dragging force, called viscous drag or viscous force, acts
tangentially on the layers of the fluid in motion and tends to destroy its motion.
Cause of viscosity
Consider a liquid moving slowly and steadily over a fixed horizontal surface.
Each layer moves parallel to the fixed surface. The layer in contact with the fixed surface is at
rest and the velocity of the every other layer increases uniformly upwards, as shown by
arrows of increasing lengths in Figure.
Consider any two adjacent layers a and b. The upper fast moving layer a tends to accelerate
the lower slow moving layer b while the slow moving layer b tends to retard the fast moving
layer a. As a result, a backward dragging tangential force F, called viscous drag comes into
play which tends to destroy the relative motion. To maintain the motion, an external force has
to be applied to overcome the backward viscous force.
Examples of viscosity
(i) When we stir a liquid contained in a beaker with a glass rod, it starts
rotating ill coaxial cylindrical layers as shown in Figure. When we
stop stirring, the speed of different layers gradually decreases and
finally the water comes to rest, showing that an internal friction
comes into play which destroys. Figure. Cylindrical the relative
motion layers in a stirred between different layers. liquid.
(ii) When we swim in a pool of water, we experience some resistance to our motion. This is on
account of viscous forces of water.
(iii) If we pour water and honey in separate funnels, water comes out readily from the hole in the
funnel while honey trickles down drop by drop very slowly. This is because honey is much
more viscous than water. The relative motion between the layers of honey is strongly
opposed.
(iv) The cloud particles fall down very slowly on account of the viscosity of air and hence seen
floating in the sky.
(v) We can walk fast in air, but not in water. This is because viscosity of air is much smaller
than that of water.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 9 of 32
Coefficient of viscosity
As shown in Figure suppose a liquid is flowing steadily in the form of parallel layers on a
fixed horizontal surface. Consider two layers P and Q at distances x and x + dx from the solid
surface and moving with velocities v and v + dv respectively. Then dv is the rate of change
of velocity with dx distance in the direction of increasing distance and is called velocity
gradient.
According to Newton, a force of viscosity F acting tangentially between two layers is
i. proportional to the area A of the layers in contact.
𝐹 ∝ 𝐴
𝑑𝑣
ii. proportional to the velocity gradient 𝑑𝑥
𝑑𝑣
𝐹 ∝
𝑑𝑥
𝑑𝑣
∴ 𝐹 ∝ 𝐴
𝑑𝑥
𝑑𝑣
𝑜𝑟 𝐹 = −𝜂 𝐴
𝑑𝑥
where 𝜂 is the coefficient of viscosity of the liquid. It depends on the nature of the liquid and
gives a measure of viscosity. Negative sign shows that the viscous force acts in a direction
opposite to the direction of motion of the liquid.
𝑑𝑣
𝐼𝑓 𝐴 = 1, 𝑎𝑛𝑑 = 1
𝑑𝑥
𝑡ℎ𝑒𝑛 𝐹=𝜂 (𝑛𝑢𝑚𝑒𝑟𝑖𝑐𝑎𝑙𝑙𝑦)
Hence coefficient of viscosity of a liquid may be defined as the tangential viscous force
required to maintain a unit velocity gradient between its two parallel layers each of unit
area.
Dimensions of 𝜼 = [𝑴𝑳−𝟏 𝑻−𝟏 ]
Units of coefficient of viscosity
(i) The CGS unit of 𝜂 is dyne s cm-2 or g cm-1 s-1 and is called poise.
The coefficient of viscosity a liquid is said to be 1 poise if a tangential force of 1 dyne
cm-2 of the surface is required to maintain a relative velocity of 1 cm s-1 between two
layers of the liquid 1 cm apart.
(ii) The SI unit of 𝜂 is N s m-2 or kg m-1 s-1 and is called decapoise or poiseuille.
The coefficient of viscosity of a liquid is said to be 1 poiseuille or decapoise if a
tangential force of 1 Nm-2 of the surface is required to maintain a relative velocity of
ms-1 between two layers of the liquid 1 m apart.
l poiseuille or 1 decapoise = 1 N s m-2
Comparison Between Viscous Force and Solid Friction
Points of similarity:
(i) Both viscous force and solid friction come into play whenever there is relative motion.
(ii) Both oppose the motion.
(iii) Both are due to molecular attractions.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 10 of 32
Points of differences:
Viscous force Solid friction
1. Viscous force is directly proportional to Solid friction is independent of the area of
the area of layers in contact. the surfaces in contact.
2. It is directly proportional to the relative It is independent of the relative velocity
velocity between the two liquid layers. between two solid surfaces.
3. It is independent of the normal reaction It is directly proportional to the normal
between the two liquid layers. reaction between the surfaces in contact.
Effect of temperature on viscosity –
(i) When a liquid is heated, the kinetic energy of its molecules increases and the
intermolecular attractions become weaker. Hence the viscosity of a liquid decreases
with the increase in its temperature.
(ii) Viscosity of gases is due to the diffusion of molecules from one moving layer to
another. But the rate of diffusion of a gas is directly proportional to the square root of
its absolute temperature, so viscosity of a gas increases with temperature as
Effect of pressure –
(i) Except water the viscosity of liquids increases with the increase in pressure. In case of
water, viscosity decreases with the increase in pressure for first few hundred
atmospheres of pressure.
(ii) The viscosity of gases is independent of pressure.
POISEUILLE'S FORMULA
The volume of a liquid flowing out per second through a horizontal capillary tube of length l,
radius r, under a pressure difference p applied across its ends is given by
𝑉 𝜋𝑝 𝑟 4
𝑄 = =
𝑡 8𝜂𝑙
This formula is called Poiseulle's formula.
Assumptions used in the derivation of Poiseuille's formula:
1. The flow of the liquid is steady and parallel to the axis of the tube.
2. The pressure is constant over any cross-section of the tube.
3. The liquid velocity is zero at the walls of the tube and increases towards the axis of the
tube.
4. The tube is held horizontal so that gravity does not influence the flow of liquid.
VISCOUS DRAG ON A BODY FALLING THROUGH A FLUID
When a body falls through a viscous fluid, the layer of the fluid in contact with the body
moves with its velocity. However, the fluid at large distance from it remains at rest. This
produces relative motion between different layers of the fluid. As a result, the body
experiences a viscous force which tends to retard its motion. This retarding force increases
with the increase in velocity of the body.
STOKES' LAW
According to Stokes' law, the backward viscous force acting on a small spherical body
of radius r moving with uniform velocity v through fluid of viscosity 𝛈 is given by
𝐹 = 6𝜋𝜂𝑟𝑣
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 11 of 32
Derivation of Stokes' law
The viscous force F acting on a sphere moving through a fluid may depend on
(i) coefficient of viscosity 𝜂 of the fluid
(ii) radius r of the spherical body
(iii) velocity v of the body
Let 𝐹 = 6𝜋𝜂𝑟𝑣 ... (1)
where k is dimensionless constant.
The dimensions of various quantities are
[F] = [MLT-2], [ 𝜂] = [ML-1T-1], [r] = [L], [v] = [LT-1]
Substituting these dimensions in equation (1), we get
[MLT-2] = [M L-1T-1]a [ L]b [LT - 1]c
= [Ma L- a + b + c T - a - c]
Equating the powers M, L and T on both sides, we get
a=1
−a + b + c = 1
−a − c = −2
On solving, a = b = c = 1
For a small sphere, k is found to be equal to 6π.
Hence F = 6πηrv
This proves Stokes' law.
Conditions under which Stokes' law is valid:
(i) The fluid through which the body moves has infinite extension.
(ii) The body is perfectly rigid and smooth.
(iii) There is no slip between the body and fluid.
(iv) The motion of the body does not give rise to turbulent motion and eddies. Hence
motion is streamlined.
(v) The size of the body is small but it is larger than the distance between the molecules of
the liquid. Thus the medium is homogeneous and continuous for such a body.
TERMINAL VELOCITY
When a body falls through a viscous fluid, it produces relative motion between its different
layers. As a result, the body experiences a viscous force which tends to retard its motion. As
the velocity of the body increases, the viscous force 𝐹 = 6𝜋𝜂𝑟𝑣 also increases. A stage is
reached, when 𝜂 the weight of the body becomes just equal to the sum of the upthrust and
viscous force. Then no net force acts on the body and it begins to move with a constant
velocity.
The maximum constant velocity acquired by a body while falling through a viscous
medium is called its terminal velocity.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 12 of 32
Expression for terminal velocity
Consider a spherical body of radius r falling through a viscous liquid of density 𝜎 and
coefficient of viscosity η· Let 𝜌 be the density of the body.
As the body falls, the various forces acting on the body are as shown in Figure. These are
(a) Weight of the body acting vertically downwards.
4
𝑊 = 𝑚𝑔 = 𝜋 𝑟 3 𝜌𝑔
3
(b) Upward thrust equal to the weight of the liquid displaced.
4
𝑈 = 𝜋 𝑟 3 𝜎𝑔
3
(c) Force of viscosity F acting in the upward direction. According to Stokes' law,
𝐹 = 6𝜋𝜂𝑟𝑣
Clearly, the force of viscosity increases as the velocity of the body increases. A stage is
reached, when the weight of the body becomes just equal to the sum of the upthrust and the
viscous force. Then the body begins to fall with a constant maximum velocity, called
terminal velocity.
When the body attains terminal velocity v,
𝑈+𝐹 =𝑊
4 4
𝜋 𝑟 3 𝜎𝑔 + 6𝜋𝜂𝑟𝑣 = 𝜋 𝑟 3 𝜌𝑔
3 3
4
6𝜋𝜂𝑟𝑣 = 𝜋 𝑟 3 (𝜌 − 𝜎)𝑔
3
2 𝑟 2 (𝜌 − 𝜎)𝑔
𝑣=
9 𝜂
This is the expression for terminal velocity.
Discussion of the result:
Figure shows how the velocity of a small sphere dropped from
rest into a viscous medium varies with time. Initially the body is
accelerated and after some time, it acquires terminal velocity v.
The terminal velocity is directly proportional to the square
of the radius of the body. That is why bigger rain drops fall
with a larger velocity compared to the smaller rain drops.
The terminal velocity is directly proportional the difference of
the densities of the body and the fluid, i.e., (𝜌 − 𝜎 ).
a. If 𝜌 > 𝜎, the body will attain terminal velocity in the downward direction.
b. If 𝜌 < 𝜎, the terminal velocity will be negative i.e., the body will rise through the fluid.
That is why, air bubble in a liquid and clouds in a sky are seen to move in the upward
direction.
c. If 𝜌 = 𝜎, the body remains suspended in the fluid.
The terminal velocity is inversely proportional to the coefficient of viscosity of the fluid.
The more viscous the fluid, the smaller the terminal velocity attained by a body.
The terminal velocity is independent of the height through which a body is dropped.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 13 of 32
STREAMLINE FLOW
When a liquid flows such that each particle of the liquid passing a given point moves
along the same path and has the same velocity as its predecessor, the flow is called
streamline flow or steady flow.
Consider the flow of the liquid along the path ABC; where A, Band C are the points inside the
𝑣𝐴 directed
liquid. If every successive particle passes through point A with constant velocity ����⃗:i
along tangent at A, then through point B with constant velocity ����⃗
𝑣𝐵 directed along tangent at
Band then through C with constant velocity ����⃗'𝑣𝐶 the flow is said to be steady, orderly or
streamlined. The path ABC along which the particles move one after another is called a
streamline. The particle velocity at a particular point remains constant with time but
velocities at different points may or may not be the same. Streamline flow is possible only if
the liquid velocity does not exceed a limiting value, called critical velocity. The fixed path
followed by an orderly procession of particles in the steady flow is called a streamline. In Fig.
the curve ABC represents a streamline. A streamline may be defined as the path, the
tangent to which at any point gives the direction of the flow of liquid at that point.
Tube of flow
A bundle of streamlines forming a tubular region is called a tube of flow. The boundary
of such a tube is always parallel to the velocity of fluid particles. No fluid can cross the
boundaries of a tube of flow, and the tube behaves somewhat like a tube. In a steady flow, the
shape of the flow tube does not change with time.
TURBULENT FLOW
When the liquid velocity exceeds a certain limiting value, called critical velocity, the liquid
flow becomes zig-zag. The path and the velocity of a liquid particle changes continuously,
haphazardly. This flow is called turbulent flow. It is accompanied by random, irregular,
local circular currents called vortices.
As shown in Fig. a jet of air striking a flat plate placed perpendicular to it causes a turbulent
flow.
Properties of streamlines:
In a steady flow, no two streamlines can cross each other. If they do so, the fluid p
article at the point of intersection will have two different directions of flow. This will
destroy the steady nature of the fluid flow.
The tangent at any point on the streamline gives the direction of velocity of fluid particle
at that point.
Greater the number of streamlines passing normally through a section of the fluid, larger
is the fluid velocity at that section.
Fluid velocity remains constant at any point of a streamline, but it may be different at
different points of the same streamline.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 14 of 32
LAMINAR FLOW
When the velocity of the flow of a liquid less than its critical velocity, the liquid flows
steadily. Each layer of the liquid slides over the other layer. It behaves as if different lamina
are sliding over one another. Such a flow is called laminar flow.
The surface obtained by joining the heads of the velocity vectors for the particles in a section
of a flowing liquid is called a velocity profile.
Velocity profile for a non-viscous liquid
In case of a non-viscous liquid, the velocity of all the particles at any section of a pipe is
same, so the velocity profile is plane as shown in Figure. (a)
Velocity profile of a viscous liquid
When a viscous liquid flows through a pipe, the velocity of layer at the axis is maximum, the
velocity decreases as towards the wall of the pipe and becomes zero for the layer in contact
with the pipe. Hence the velocity profile for a viscous liquid is parabolic, as shown in Fig. (b)
Critical velocity
The critical velocity of a liquid is that limiting value of its velocity of flow upto which
the flow is stream. lined and above which the flow becomes turbulent.
The critical velocity 𝑣𝑐 of a liquid flowing through a tube depends on
(i) coefficient of viscosity of the liquid (𝜂)
(ii) density of the liquid (𝜌)
(iii) diameter of the tube (D)
𝐿𝑒𝑡 𝒗𝒄 = 𝒌 𝜼𝒂 𝝆 𝒃 𝑫𝒄
where k is a dimensionless constant. Writing the above equation in dimensional form, we get
[M0LT-1 ] = [ML-1T-1]a [ML-3] b [L] c
[MOL r 1 l = [ M" + b L- a - 3b + CT - a l
Equating powers of M, L and T, we get
𝑎+𝑏 =0
− 𝑎 – 3𝑏 + 𝑐 = 1
−𝑎 = − 1
On solving, we get a = 1, b = -1, c = - 1
∴ 𝑣𝑐 = 𝑘𝜂𝜌−1 𝐷−1
𝑘𝜂
𝑣𝑐 =
𝜌𝐷
Clearly, the critical velocity vc will be large if 11 is large, and p and Dare small. So we can
conclude that
i. The flow of liquids of higher viscosity and lower density through narrow pipes tends to
be streamlined.
ii. The flow of liquids of ·rower viscosity and higher density through broad pipes tends to
become turbulent, because in that case the critical velocity will be very small.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 15 of 32
REYNOLD'S NUMBER
It is dimensionless parameter whose value decides the nature of flow of a liquid through
a pipe. It is given by
𝜌𝑣𝐷
𝑅 =
𝜂
where 𝜌 = density of the liquid
v = velocity of the liquid
η = coefficient of viscosity of the liquid
D = diameter of the pipe.
Importance of Reynold's number
If Re lies between 0 and 2000, the liquid flow is streamlined or laminar. If Re > 3000, the
liquid flow is turbulent. If Re lies between 2000 and 3000, the flow of liquid is unstable, it
may change from laminar to turbulent and vice-versa. The exact value at which turbulence
sets in a fluid is called critical Reynold's number.
Physical significance of Reynold's number
Reynold's number represents the ratio of the inertial force per unit area to the viscous force
per unit area.
IDEAL FLUID
The motion of real fluids is very complicated. To understand fluid dynamics in a simpler
manner, we assume that the fluid is ideal. An ideal fluid is one which is non-viscous,
incompressible, and its flow is steady and irrotational. Thus an ideal fluid has the
following features connected with its flow:
(i) Steady flow. In a steady flow, the fluid velocity at each point does not change with
time, either in magnitude or direction.
(ii) Incompressible flow. The density of the fluid remains constant during its flow.
(iii) Non-viscous flow. The fluid offers no internal friction. An object moving through this
fluid does not experience a retarding force.
(iv) Irrotational flow. This means that there is no angular momentum of the fluid about
any point. A very small wheel placed at any point inside such a fluid does not rotate
about its centre of mass.
EQUATION OF CONTINUITY
Consider a non-viscous and incompressible liquid flowing steadily between the sections A
and B of a pipe of varying cross-section.
Let a1 be the area of cross-section, v1 fluid velocity, p1 fluid :density at section A ; and the
values of corresponding quantities at section B be a2 , v2 and p2.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 16 of 32
As m = Volume × density
= Area of crosssection × length × density
∴ Mass of fluid that flows through section A in time ∆t,
m1 = a1 v1 ∆t ρ1
Mass of fluid that flows through section B in time ∆t,
m2 = a2 v2 ∆t ρ2
By conservation of mass,
m1 = m2
a1 v1 ∆t ρ1 = a2 v2 ∆t ρ2
As the fluid is incompressible, so ρ1 = ρ2 , and hence
a1 v1 = a1 v1 or av = constant.
This is the equation of continuity. It states that during the streamlined flow of the non-
viscous and incompressible fluid through a pipe of varying cross-section, the product of
area of cross-section and the normal fluid velocity (av) remains constant throughout the
flow.
ENERGY OF A FLUID IN A STEADY FLOW
(i) Kinetic energy -
The energy possessed by a liquid by virtue of its motion is called its kinetic energy.
1
𝐾. 𝐸. = 𝑚𝑣 2
2
where m is the mass of the liquid and v is the velocity of the liquid.
The kinetic energy per unit weight of the liquid is known as the velocity head.
𝑣2
𝑉𝑒𝑙𝑜𝑐𝑖𝑡𝑦 ℎ𝑒𝑎𝑑 =
2𝑔
(ii) Potential energy –
The energy possessed by a liquid by virtue of its position above the earth 's surface is
called its potential energy.
P. E. = mgh
where h is the average height of the liquid from the ground level.
The potential energy per unit weight of the liquid is known as the potential head.
mgh
Potential head = =h
mg
(iii) Pressure energy –
The energy possessed by n liquid by virtue of its pressure is called its pressure energy. A
liquid under pressure can do work and so possesses energy.
For example, a liquid in a cylinder can drive a piston as shown in Figurer. Let P be the
pressure exerted by the liquid on a frictionless piston of area a.
Suppose the piston moves through distance x under the pressure P.
The work done is
W = Force × distance = Pressure × area × distance
= Pax = PV
where V = ax = volume swept by the piston.
This work done is stored as the pressure energy liquid of volume V.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 17 of 32
: . Pressure energy of volume V = PV
Pressure energy per unit volume
PV
== P = Excess pressure
V
PV P
Pressure energy per urut mass = =
m ρ
Pressure energy per unit weight of the liquid is called pressure head.
P
Pressure head =
ρg
BERNOULLI'S PRINCIPLE
Bernoulli's principle is based on the law of conservation of energy and applied to ideal fluids.
It states that the sum of sure energy per unit volume, kinetic energy per unit volume and
potential energy per unit volume of an incompressible, non-viscous fluid in a streamlined
irrotational flow remains constant at every cross-section throughout the liquid flow.
Mathematically, it can be expressed as
1
P + ρv 2 + ρgh = constant
2
where, P represents for pressure energy per unit volume;
1
2
𝜌𝑣 2 for kinetic energy per unit volume and
𝜌𝑔ℎ for potential energy per unit volume
The Swiss physicist Daniel Bernoulli developed this relationship in 1738.
Proof –
Consider an ideal fluid having streamline flow through a pipe of varying area of cross-section
as shown in figure.
Let P1 , a1 , h1 , v1 and P2 , a2 , h2 , v2 be the pressure, area of cross-section, height and velocity
of flow at points A and B, respectively. Force acting on fluid at point A = P1 a1
Distance travelled by fluid in one second at point A
= v1 × 1 = v1
Work done per second on the fluid at point A
= Force × distance travelled by fluid in one second. W1 = P1 a1 × v1
Similarly, work done per second by the fluid at point B,
W2 = P2 a2 × v2
Net work done on the fluid by pressure energy,
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 18 of 32
W = P1 a1 v1 − P2 a2 v2
m
But, a1 v1 = a2 v2 = ρ
(i.e. equation of continuity)
∴ Net work done on the fluid by the pressure energy,
P1 m P2 m
W= � ρ
− ρ
�
Total work done by the pressure energy on the fluid increases the kinetic energy and potential
energy of the fluid, when it flows from A to B.
∴ Increase in potential energy of fluid
= KE at B − KE at A
1 1
= 2 mv22 − 2 mv12 [∵ a1 > a2 ∴ v2 > v1 ]
Similarly, total increase in potential energy = mgh2 − mgh1 According to work energy
theorem, work done on the fluid is equal to change in the energy of fluid.
i.e. work done by the pressure energy = total increase in energy
P1 m P2 m 1 1
∴ ρ
− ρ
= (mgh2 − mgh1 ) + �2 mv22 − 2 mv12 �
P1 − P2 1 1
� � = (gh2 − gh1 ) + 2 v22 − 2 mv12
ρ
P1 1 P2 1
Or + 2 v12 + gh1 = + 2 v22 + gh2
ρ ρ
P 1
Hence, ρ
+ gh + 2 v 2 = constant
1
P + 2 ρv 2 + ρgh = constant …(i)
Dividing both sides of Eq. (i) by ρg, we get
P v2 constant
ρg
+ h + 2g = ρg
= new constant …(ii)
P v2
Here, ρg is called pressure head, h is called gravitational head and 2g is called velocity head.
Equation (ii) enable us to state Bernoulli's theorem in the streamline flow of an ideal liquid as
the sum of pressure head, gravitational head and velocity head is always constant. If the fluid
is flowing through a horizontal tube, two ends of the tube are at the same level. Therefore,
there is no gravitational head (level difference), i.e. h = 0.
P 1 1
ρ
+ 2 v 2 = P + 2 ρv 2 = constant
This shows, if P increases, v decreases and vice-versa. Thus, Bernoulli's theorem also states
that in the streamline flow of an ideal liquid through a horizontal tube, the velocity increases
where pressure decreases and vice-versa. This is also called Bernoulli’s principle.
Limitations of Bernoulli's equation
1. Bernoulli's equation ideally applies to fluids with zero viscosity or non-viscous fluids.
2. The fluids must be incompressible, as the elastic energy of the fluid is also not taken into
consideration.
3. Bernoulli's equation is applicable only to streamline flow of a fluid. It is not valid for non-
steady or turbulent flow.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 19 of 32
TORRICELLI'S LAW OF EFFLUX
Speed of efflux. The word efflux means the outflow of a fluid. As shown in Figure, consider
a tank containing a liquid of density 𝜌 with a small hole on its side at a height y1 from the
bottom. Let y2 be the height of the liquid surface from the bottom and P be the air pressure
above the liquid
If A1 and A2 are the cross-sectional areas of the side hole and the tank respectively, and v1 and
v2 are the liquid velocities at points 1 and 2, then from the equation of continuity, we get
A
v1 = v2 ⇒ v2 = A1 v1
2
As A2 ≫ A1 , so the liquid may be taken at rest at the top, i.e., v2 ≈ 0. Applying Bernoulli's
equation at points 1 and 2, we get
1
Pa + ρv12 + ρgy1 = P + +ρgy2
2
1 2
ρv = ρg(y2 − y1 ) + (P − Pa )
2 1
If we take (y2 − y1 ) = h then
1 2
ρv = ρgh + (P − Pa )
2 1
2(P − Pa )
v1 = �2gh +
ρ
Special cases :
(i) When P ≫ Pa , the term 2gh may be ignored.
2(P − Pa )
v1 = �
ρ
Thus the speed of efflux is determined by container pressure P. Such a situation exists in
rocket propulsion.
(ii) When the tank is open to the atmosphere.
P = Pa and v1 = �𝟐𝐠𝐡
Thus, the velocity of efflux of a liquid is equal to the velocity which a body acquires in
falling freely from the free liquid surface to the orifice. This result is called Torricelli's
law.
APPLICATION OF BERNAULLI’S THEOREM
ATOMIZER
The working of an atomizer which is used to spray
liquids is based on Bernoulli's principle.
Fig. shows the essential parts of an atomizer. When
the rubber balloon is pressed, the air rushes out of
the horizontal tube B decreasing the pressure to P,
which is less than the atmospheric pressure P1 in the
container. As a result, the liquid rises up in the
vertical tube A. When it collides with the high speed
air in tube B, it breaks up into a fine spray.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 20 of 32
MAGNUS EFFECT [CURVED PATH OF A SPINNING BALL]
When a ball is thrown horizontally with a large velocity and at the same time given a twisting
motion to cause a spin, it deviates from its usual parabolic trajectory of spin free motion. This
deviation can be explained on the basis of Bernoulli's principle.
When the ball spins about an axis perpendicular to its horizontal motion, it carries with itself
an air of layer due to viscous drag. The streamlines around it are in the form of concentric
circles, as shown in Figure (a).When the ball moves forward with velocity v, the air ahead of
the ball rushes backward with velocity v to fill the space left empty by the ball. Thus the
streamlines in air due to translatory motion of the ball are of the form shown in Figure (b).
The layer above the ball moves in a direction opposite to that of the spinning ball, so the
resultant velocity decreases and hence pressure increases in accordance with Bernoulli's
principle. The layer below the ball moves in the direction of spin, the resultant velocity
increases and hence pressure decreases. Due to the difference of pressure on the two sides of
the ball, the ball curves downwards in the direction of spin, as shown in Figure (c).
The difference in lateral pressure, which causes a spinning ball to take a curved path
which is convex towards the greater pressure side, is called magnus effect. This effect
was first noticed by German scientist H.G. Magnus in the mid-nineteenth century. The
rougher the surface, the thicker is the layer of air dragged along by the spinning ball, and
more curved the path.
AEROFOIL : LIFT OF AN AIRCRAFT WING
Aerofoil is the name given to a solid object shaped to provide an upward vertical force
as it moves horizontally through air. This upward force(dynamic lift) makes aeroplanes fly.
As shown in Figure, the cross-section of the wing of
an aeroplane looks like an aerofoil. The wing is so
designed that its upper surface is more curved (and
hence longer) than the lower surface and the front
edge is broader than the rear edge. As the aircraft
moves, the air moves faster over the upper surface of
the wing than on the bottom. According to Bernoulli's
principle, the air pressure above the upper surface
decreases below the atmospheric pressure and that on
the lower surface increases above the atmospheric
pressure.
The difference in pressure provides an upward lift, called dynamic lift, to the aircraft.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 21 of 32
BLOOD FLOW AND HEART ATTACK
In persons suffering with advanced heart condition, the artery gets constricted due to the
accumulation of plaque on its inner walls. In order to drive the blood through this
constriction, a greater demand is placed on the activity of the heart. The speed of blood flow
increases in this region. From Bernoulli's principle, the inside pressure drops and the artery
may collapse due to external pressure. The heart exerts further pressure to open this artery
and forces the blood through. As the blood rushes through the opening, the internal pressure
once again drops leading to a repeat collapse. This phenomenon is called vascular flutter
which can be heard on a stethoscope. This may result in a heart attack.
Blowing off the roof during wind storm
During certain wind storm or cyclone, the roofs of some houses are blown off without
damaging the other parts of the house. The high wind blowing over the roof creates a low
pressure P2 in accordance with Bernoulli's principle. The pressure P1 below the roof is equal
to the atmospheric pressure which is larger than P2. The difference of pressure (P1 - P2) causes
an upward thrust and the roof is lifted up. Once the roof is lifted up, it is blown off with the
wind.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 22 of 32
INTER MOLECULAR FORCES
1. Cohesion − The force of attraction between molecules of the same substance is called
the force of cohesion.
The force of cohesion is maximum in solids, lesser in liquids and least in gases. It is for this
reason that the solids have definite shape and resist all deforming forces.
Illustration:
(i) The solids exhibit their rigid character and definite shape due to strong force of cohesion
between their molecules. The presence of a strong force of cohesion between the
molecules of a solid can be demonstrated by placing two blocks of steel together after
making their surfaces sufficiently plain. If we try to pull them apart, a large force will be
required to do so. However, in liquids, the force of cohesion is very small and almost
negligible in gases.
(ii) In general, we cannot adhere two pieces of solid simply by pressing them together. The
reason is that ordinary pressure cannot bring the molecules of the two pieces so close
(10-9 metre) that cohesive forces may become effective between them. But if their
surfaces in contact are melted by heating, the molecules in the liquid state fill up the
space between the solid surfaces. Then, on cooling, the surfaces adhere together. This is
the process to join metals by welding.
(iii) It is due to large force of cohesion between mercury molecules that it does not wet the
wall of the container.
(iv) It is due to the cohesive force that two drops of a liquid when brought in mutual contact
coalesce into one.
(v) It is difficult to separate two sticky plates of glass wetted with water because quite a
large force has to be applied against the cohesive force between the molecules of water.
The definite shape of solid substances is also due to the cohesive force present between
its molecules.
2. Adhesion − The force of attraction between the molecules of the different substances is
called the force of adhesion.
The examples of adhesion are the sticking of glue to the wood, paint to the wall, starch to the
fingers, chalk particles to the black board, water to its container, etc. The force of adhesion
between the lubricating oil and the parts of a machine plays an important role in avoiding the
wear and the tear of the machine.
Illustration:
(i) A piece of paper sticks to another due to large force of adhesion between the paper and
the gum molecules.
(ii) Paint sticks to wood (and other surfaces) due to large force of adhesion between the
surface of wood and paint.
(iii) Because of the large force of adhesion between cement and bricks, cement is used in
construction work.
(iv) In order to dry the wet plate it should be wiped by a substance whose adhesion for water
molecules is greater than of glass, for example rough dry cloth. Silken and nylon cloths
cannot be used to dry wet glass plate because their adhesion for water is less.
(v) Because the particles of chalk stick to the blackboard due to large force of adhesion, we
are able to write on a blackboard with a piece of chalk.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 23 of 32
Water wets the glass surface, but mercury does not:
The adhesive force between water molecules and glass molecules is greater than the cohesive
force between the molecules of water. Hence, when water is poured on glass, the water
molecules cling with the glass molecules and the glass surface is wetted. On the other hand,
the adhesive force between mercury molecules and glass molecules is less than the cohesive
force between mercury molecules. Hence mercury molecules do not cling with glass
molecules, that is, mercury does not wet the glass. If, however, the glass surface is greasy,
then water also does not wet the glass because the adhesive force between water and grease is
less than the cohesive force between water molecules themselves.
The adhesive force between oil and water is less than the cohesive force of water, but greater
than the cohesive force of oil. Therefore, a water drop poured on the surface of oil contracts
to take the form of a globule, while a drop of oil poured on the surface of water spreads to a
large area in the form of a thin film.
The adhesive force between ink and paper is greater than the cohesive force of ink. That is
why ink sticks on paper. Writing on blackboard by chalk is also possible due to adhesive
force.
SURFACE TENSION
It is found that when a liquid is free from the external forces (such as gravity), it always takes
the shape of a spherical drop. It is because, for a given volume, a sphere has the least surface
area. It means that the surface of every liquid has always a tendency to have least surface area
and in this respect, it behaves like a stretched membrane having a tension in all directions
parallel to the surface. This tension in the surface of a liquid is called surface tension.
Thus, “surface tension is that property of a liquid by virtue of which, it behaves like an elastic
stretched membrane with a tendency to contract, so as to occupy a minimum surface area”.
Imagine a line AB drawn on the free surface of a liquid as shown in figure. In order to have
the minimum surface area, the two portions of the liquid surface on either side of the line AB
have tendency to draw away from each other. Imagine that the surface film is cut along the
line AB.
Then, the film on the either side of the line will contract. This shows
that there are forces acting on the either side of the line, which tend to
pull the liquid surface apart along this line, the film being prevented
from breaking into two parts due to the force of attraction between
molecules. The magnitude of such a force per unit length of the line
drawn on the surface of the liquid gives the measure of the surface
tension.
Hence, “the surface tension of a liquid can be measured as the force per unit length on
an imaginary line drawn on the liquid surface, which acts perpendicular to the line on
its either side at every point and tangentially to the liquid surface”.
SURFAGE ENERGY of a Liquid
When the surface area of a liquid is increased, the molecules from the interior rise to the
surface. This requires work against the force of attraction of the molecules just below the
surface. This work is stored in the form of potential energy in the newly formed surface.
Besides this, there is some cooling due to the increase in the surface area. Therefore, heat
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 24 of 32
flows into the surface from the surroundings to keep its temperature constant and is added to
its energy. Thus, the molecules in the surface have some additional energy due to their
position. This additional energy per unit area of the surface is called 'surface energy of the
liquid'. So,
Surface energy of a liquid is defined as “the amount of work done in increasing the area of the
surface film through unity”.
𝒘𝒐𝒓𝒌 𝒅𝒐𝒏𝒆 𝒊𝒏 𝒊𝒏𝒄𝒓𝒆𝒂𝒔𝒊𝒏𝒈 𝒕𝒉𝒆 𝒔𝒖𝒓𝒇𝒂𝒄𝒆 𝒂𝒓𝒆𝒂
𝒔𝒖𝒓𝒇𝒂𝒄𝒆 𝒆𝒏𝒆𝒓𝒈𝒚 =
𝒊𝒏𝒄𝒓𝒆𝒂𝒔𝒆 𝒊𝒏 𝒔𝒖𝒓𝒇𝒂𝒄𝒆 𝒂𝒓𝒆𝒂
Relation Between Surface Energy and Surface Tension
Consider a rectangular frame ABCD made of a wire, such that the cross-piece LM can just
slide. Dip the wire frame in the soap solution, so that a film is formed over the frame [see
figure]. Due to the surface tension, the film will have a tendency to shrink and thereby, the
cross-piece LM will be pulled in inward direction. However, the cross-piece can be held in
this position under a force F, which is equal and opposite to the force acting on the cross piece
LM all along its length due to surface tension in the soap film. If T is the surface tension of
the soap solution, then
𝐹 = 𝑇 × 2𝑙
Here, 𝑙 is length of the cross-piece LM. The length
of the cross-piece has been taken as 2𝑙 for the reason
that the film has got two free surfaces.
Suppose that the cross-piece LM is moved through a
small distance x, so as to take the position L′ M ′ . In
this process, area of the film increases by 2 𝑙 𝑥 (on
the two sides) and to do so, the work done is given by
𝑊 = 𝐹 × 𝑥 = (𝑇 × 2 𝑙) × 𝑥
This amount of work has been done in increasing area of surface film by an amount 2𝑙 x.
Therefore, by definition,
(𝑻 × 𝟐𝒍) ×𝒙
𝒔𝒖𝒓𝒇𝒂𝒄𝒆 𝒆𝒏𝒆𝒓𝒈𝒚 = =𝑻
𝟐𝒍×𝒙
Hence, the surface tension of a liquid is numerically equal to its surface energy.
SOME OBSERVED PHENOMENA ON BASIS OF SURFACE TENSION
1. Hair of a brush, when dipped in water spread out; but as soon as it is taken out, its hair cling
together.
The reason is that when the brush is inside water, there is water all around its hair. As water
has no free surface, the tips of the hair of the brush remain spreaded. But when the brush is
taken out from the water, a thin water film is formed at the tips of the hair. The surface of the
water film formed contracts due to surface tension and the hair cling together.
2. Liquid in small quantities free from external forces like gravity etc are found spherical in shape.
For example, rain drops or a globule of mercury placed on clean glass plate.
The reason is that due to surface tension, the liquid tries to acquire minimum surface area. As
for given volume, a sphere has minimum surface area, the liquid assumes the shape of a drop.
This is so, because the liquid tries to assume a shape so that surface area is minimum and
since for a given volume; a sphere has least surface area, a liquid in small quantity acquires
spherical shape.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 25 of 32
3. Take a frame of wire and dip it in soap solution and take it out.
A soap film will be formed in the frame. Place a loop of wet
thread gently on the film. It will remain in the form, we place it
on the film [Figure (a)]. Now, pierce the film with a pin at any
point inside the loop. The loop immediately takes the circular
form as shown in [Figure (b)].
On piercing the soap film within the loop, the unbalanced outward pull acts due to surface
tension at every point of the loop. The portion of the liquid film left between the frame of
wire and, the loop of thread contracts to a minimum possible area, for which it is necessary
that empty portion of the loop of thread should enclose maximum area. Now, for a given
parameter, a circle has maximum surface area. So, due to tension in the liquid film, the loop
of the thread assumes a circular shape.
4. Take a greased needle on a piece of blotting paper and place it gently over the water
surface. Blotting paper soaks water and soon sinks down but the needle keeps floating.
The reason is that force due to surface tension of the water equal
to T per unit length acts along tangent to the curved surface and
the resultant of the vertical components of such forces balances
the weight W of the needle, acting vertically downwards. A slight
depression is seen below the needle, indicating that the water
surface behaves like a stretched membrane.
5. If a small irregular piece of camphor is floated on the surface of pure water, it does not
remain steady but dances about on the surface. This is because, irregular shaped camphor
dissolves unequally and decreases the surface tension of the water locally. The unbalanced
forces make it move haphazardly in different directions.
6. When a molten metal is poured into water from a suitable height, the falling stream of
metal breaks up and the detached portions of the liquid in small quantity acquire the
spherical shape. In order to form lead shots, the melted lead is allowed to fall in water by
spraying it from some height. During its fall the melted lead, due to surface tension, acquires
spherical shape and on entering water it becomes solid. Thus, small spherical lead shots are
formed.
7. Oil spreads on cold water. However, it may remain as a drop on hot water. It is due to the
reason that the surface tension of oil is less than that of the cold water but greater than that of
the hot water.
8. Bigger bubbles can be formed from the soap solution than from water. Bubbles formed
from pure water break at a very early stage due to surface tension of water. The soap solution
has a comparatively much lower surface tension and so bigger bubbles of the solution can be
formed.
9. Soap helps in cleaning the clothes. The soap reduces the surface tension of the solution.
Hence, a drop of soap solution wets a larger surface area of the cloth in comparison to a drop
of pure water. Thus, soap solution enters in those fine holes where pure water cannot reach
and bring out the dirt particles with it (the adhesive force between the solution and the dirt is
greater than the cohesive force of the solution itself). Hence the soap solution cleans the cloth
much more than pure water. If the soap solution is heated, its surface tension is further
lowered and it can clean even more.
10. The ends of a glass tube become rounded on heating. When glass is heated it melts to a
liquid. The surface of this liquid tends to have a minimum area. We know that for a given
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 26 of 32
volume, the area of the surface of a sphere is minimum. Hence, the melted glass tends to
assume a spherical shape.
11. Spraying results in coldness. When a liquid is sprayed into a large number of droplets, the
surface area of the liquid increases. In this process the molecules from the interior of the
liquid rise to the surface of the drops, doing work against the cohesive force. This results in a
decrease in the internal energy of the molecules. Consequently the temperature of the drops
falls.
12. Liquid-drop Model of Nucleus. In a liquid drop the molecules of the liquid are bounded by
cohesive forces. These forces cause surface tension in the liquid due to which the drop
acquires spherical shape. In an atomic nucleus also, there are particles named protons and
neutrons between which short range (nuclear) attractive forces act and keep the particles
bound together. In the liquid-drop model of the nucleus, it has been assumed that, like a
liquid drop, in the nucleus also there is surface-tension due to which its shape is spherical.
If we deform a liquid drop by pushing it slightly, then due to surface tension it again becomes
spherical. But if we push the drop with a force it may be elongated and divided into two
parts. Similarly, in nuclear fission, when an external neutron strikes the nucleus, the nucleus
is deformed. The nuclear forces tend to bring the nucleus back into the spherical shape, while
the (electrostatic) repulsive-forces between the protons tend to break the nucleus. If the
deformation is small, the nucleus acquires its spherical shape due to the nuclear forces
[Figure. (a)]. But if the deformation is large, the nucleus is broken up into two parts due to
electrostatic forces [Figure (b)].
13. Some other facts:
I. Medicines used for washing wounds, as dettol, have a surface tension lower than
water. Hence they reach the fine cavities formed in the wound.
II. The surface tension of the tooth-paste scum is also less so that it spreads quickly on the
full areas of teeth and cleans them.
III. Hot soup is more tasteful than the cold one because the surface tension of the hot soup
is less than that of the cold and so it spreads over a larger area of the tongue.
SHAPE OF LIQUID MENISCUS IN GLASS TUBE
When a liquid is brought in contact with a solid surface, the surface of the liquid becomes
curved near the place of contact. The nature of the curvature or meniscus (concave or
convex) depends upon the relative magnitudes of the cohesive force between the liquid
molecules and the adhesive force between the molecules of the liquid and those of the solid.
In Figure (a), water is shown to be in contact with the wall of a glass tube. Let us consider a
molecule A on the water surface near the glass. This molecule is acted upon by two forces of
attraction:
(i) The resultant adhesive force P, which acts on A due to the
attraction of glass molecules near A. Its direction is perpendicular
to the surface of the glass.
(ii) The resultant cohesive force Q, which acts on A due to the
attraction of neighbouring water molecules. It acts towards the
interior of water.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 27 of 32
The adhesive force between water molecules and glass molecules is greater than the cohesive
force between the molecules of water. Hence, the force P is greater than the force Q. Thus
their resultant R will be directed outwards from water [Figure (a)].
In Figure (b), mercury is shown to be in contact with the
wall of a glass tube. The cohesive force between the
molecules of mercury is far greater than the adhesive force
between the mercury molecules and the glass molecules.
Hence, in this case, the force Q will be much greater than
the force P and their resultant R will be directed towards the
interior of mercury.
The resultant force R acts on all the molecules on the surface of water or mercury. For the
molecules more and more away from the wall, the adhesive force P goes on decreasing while
the cohesive force Q becomes more and more vertical. Consequently, the resultant R also
becomes more and more vertical. In the middle of the surface, P becomes zero and Q becomes
vertical. Hence, the resultant R becomes exactly vertical [Figure c, d].
(c) (d)
If the surface of the liquid is in equilibrium, the resultant force acting on any molecule
in the surface must be perpendicular to the surface. Hence the liquid surface sets itself
perpendicular to the resultant force everywhere. This is why the water surface assumes
a concave shape while the mercury surface assumes a convex shape in a glass tube. In
either case the resultant force in the middle is vertical and the surface there is horizontal
[Figure c, d].
Angle of Contact
When the free surface of a liquid comes in
contact of a solid, it becomes curved near the
place of contact. The angle inside the liquid
between the tangent to the solid surface and
the tangent to the liquid surface at the point
of contact is called the 'angle of contact' for
that pair of solid and liquid.
The angle of contact for those liquids which wet the solid is acute. It is zero for pure water
and clean glass; for ordinary water and glass it is about 8°. The liquids which do not wet
the solid have obtuse angle of contact. For mercury and glass the angle of contact is 135°.
The angle of contact for water and silver is 90°. Hence, in a silver vessel the surface of
water at the edges also remains horizontal.
Factors affecting Angle of Contact –
(i) Increases with increase in temperature and
(ii) Decreases on adding impurities to the liquid.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 28 of 32
PRESSURE DIFFERENCE ACROSS A CURVED LIQUID SURFACE
The surface of a liquid may be concave, convex or plane. The force due to surface tension on
molecule M in the surface of the liquid acts tangentially to the surface in all directions.
(a) When the surface is concave − If the surface of the liquid is concave, then due to surface
tension, the molecule M will experience a net outward force normal to the surface [Figure
(a)]. The liquid molecule will remain in equilibrium, if the pressure on the concave side i.e.
on the side of the liquid vapour is more.
(b) When the surface is convex − If the surface is convex, then due to surface tension, the
molecule M will experience a net inward force normal to the surface [Figure (b)]. The liquid
molecule will be in equilibrium, if the pressure on the concave side i.e. on the side of the
liquid is more.
(c) When the surface is plane − When the free surface of a liquid is plane, the molecule M is
pulled equally in all directions and the liquid molecule experiences no net inward or outward
force [Figure (c)]. In other words, there is no extra pressure on the two sides of a plane liquid
surface due to surface tension.
(a) (b) (c)
From this discussion, it follows that “a curved surface will be in equilibrium, only if
pressure on the concave side is greater than the pressure on the convex side of the
curved surface”.
EXCESS OF PRESSURE INSIDE A LIQUID DROP AND A BUBBLE
Due to the property of the surface tension, the small drops and bubbles have spherical shapes,
it implies that their spherical surfaces must be possessing minimum surface area.
(i) Excess Pressure inside a Liquid Drop:
Let us consider a liquid drop of radius R of a liquid of surface tension
T. The molecules of the liquid in the surface of the drop experience a
resultant force due to surface tension acting normally inwards.
Therefore, the pressure inside the drop must be greater than the
pressure outside it. The excess pressure inside the drop produces a
force acting outwards which balances the surface tension force and
keeps the drop in equilibrium.
Initial surface area = 4 π R2
Final surface area = 4 π (R + dR)2
= 4 π (R2 + 2RdR + dR2 )
= 4 π R2 + 8πRdR
dR2 is neglected as it is small.
Increase in surface area = 4 π R2 + 8πRdR − 4 π R2 = 8πRdR
Work done in enlarging the drop = Increase in surface energy
= Increase in surface area × surface tension
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 29 of 32
= 8 π R dR T
But work done = Force × Distance
= Pressure × Area × Distance
= p × 4 π R2 × dR
Hence,
p × 4 π R2 × dR = 8 π R dR T
Excess pressure,
2T
p=
R
Excess pressure inside a soap bubble
Increase in surface area = 8 π R dR
But a soap bubble has air both inside and outside, so it has two free surfaces.
Effective increase in surface area
= 2 × 8 π R dR = 16 π R dR
Work done in enlarging the soap bubble
= Increase in surface energy
= Increase in surface area × Surface tension
= 16 π R dR T
But,
work done = Force × Distance
= Pressurex Area × Distance
= p × 4 π R2 × dR
Hence,
p × 4 π R2 × dR = 16 π R dR T
Excess pressure,
4T
p=
R
CAPILLARITY
When a glass capillary tube open at both ends is dipped vertically in water, the water rises up
in the tube to a certain height above the water level outside the tube. The narrower the tube,
the higher is the rise of water [Figure (a)]. On the other hand, if the tube is dipped in mercury,
the mercury is depressed below the outside level [Figure (b)]. ·The phenomenon of rise or
depression of liquids in a capillary tube is called 'capillarity'. The liquids which wet glass (for
which the angle of contact is acute) rise up in the capillary tube, while those which do not wet
glass (for which the angle of contact is obtuse) are depressed down in the capillary.
Explanation: The phenomenon of capillarity arises due to the surface tension of liquids.
When a capillary tube is dipped in water, the water meniscus inside the tube is concave. The
pressure just below the meniscus is less than the pressure just above it by 2T/R, where T is
the surface tension of water and R is the radius of curvature of the meniscus. The pressure on
the surface of water is atmospheric pressure P.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 30 of 32
(a) (b) (c) (d)
The pressure just below the 'plane' surface of water outside the tube is also P, but that just
below the meniscus inside the tube is P − (2T/R) [Figure (a)]. We know that pressure at all
points in the same level of water must be the same. Therefore, to make up the deficiency of
pressure, 2T/R below the meniscus, water begins to flow from outside into the tube. The
rising of water in the capillary stops at a certain height h . In this position the pressure of the
water column of height h becomes equal to 2T/R that is,
2𝑇
ℎ𝜌𝑔 =
𝑅
where ρ is the density of water and g is the acceleration due to gravity. If r be the radius of
the capillary tube and θ the angle of contact of water-glass, then the radius of curvature R of
the meniscus is given by
𝑟
𝑅=
cos 𝜃
2𝑇
Thus ℎ𝜌𝑔 = 𝑟
�cos 𝜃
2𝑇 cos 𝜃
ℎ=
𝑟𝜌𝑔
This shows that as r decreases, h increases, that is, narrower the tube, greater is the height to
which the liquid rises in the tube.
Rising of Liquid in a capillary tube of insufficient length -
Suppose a liquid of density ρ and surface tension T rises in a capillary tube to a height h.
Then
2𝑇
ℎ𝜌𝑔 =
𝑅
where R is the radius of curvature of the liquid meniscus in the tube. From this we may write
2𝑇
h R = 𝜌𝑔 = constant (for a given liquid).
When the length of the tube is greater than h, the liquid rises in the tube to a height so as to
satisfy the above relation. But if the length of the tube is less than h, say h' then the liquid
rises up to the top of the tube and then spreads out until its radius of curvature R increases to
R’, such that
2𝑇
ℎ′𝑅′ = ℎ𝑅 =
𝜌𝑔
It is clear that liquid cannot emerge in the form of a fountain from the upper end of a short
capillary tube.
Some practical examples of capillarity −
1. The water given to the fields rises in the innumerable capillaries formed in the stems of
plants and trees and reaches the branches and the leaves.
2. The kerosene oil in lanterns and the melted wax in a candle, rise in the capillaries formed in
the cotton wick and burns.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 31 of 32
3. If one end of a towel is dipped in water filled in a vessel, then the water begins to rise in the
innumerable holes (which act like capillaries) formed in between the threads of the towel
and ultimately the entire towel is wetted.
4. Coffee powder is easily soluble in water because water immediately wets the fine granules
of coffee by the action of capillarity.
5. The action of blotting paper also depends upon capillarity. Blotting paper is porous. When
it is placed on wet ink, the ink rises in its fine holes. That is why we cannot write on
blotting paper. Also, it is for this reason that wet glass plate can be wiped by a towel, but
not by silk or nylon.
6. Writing nib is split in the middle so that a fine capillary is formed in it. When it is dipped in
ink, the ink rises in the capillary.
7. The farmers plough their fields after rains so that the capillaries formed in the soil are
broken and the water remains in the lower layers of the soil. This water is taken up by
plants. If ploughing is not done, the water of the lower layers will rise through the
capillaries in the soil and evaporate.
RISE OF LIQUID IN A CAPILLARY (ASCENT FORMULA)
Suppose a cleaned capillary tube of glass having a uniform bore of radius r is dipped in a
liquid (say water) which rises to a height h above the level outside the tube. The liquid
meniscus AEB in the tube is concave upward (Figure-a). Let T be the surface tension of the
liquid.
The liquid meniscus in the tube is along a circle of circumference 2 π r (shown by the dotted
line) which is in contact with the glass. Due to the surface tension of liquid, a force equal to
T per unit length acts at all points of the circle. If the angle of contact for liquid-glass be θ,
then this force is directed inward at an angle θ from the wall of the tube. The wall of the tube
also exerts an equal (reactionary) force T per unit length on the circumference of the liquid
meniscus. This force, which is directed outward, can be resolved into two components; T
cos θ per unit length acting vertically upward and T sin θ per unit length acting horizontally
outward. Considering the entire circumference 2π r, for each horizontal component T sin θ
there is an equal and opposite component and the two neutralise each other. The vertical
components being in the same direction are added up to give a total upward force 2πr × T
cos θ (Figure-b). It is this force which supports the weight of the liquid column so raised.
In the capillary tube, the curved surface AEB may be assumed hemispherical whose radius
may be taken equal to the radius r of the capillary tube.
Now, the volume of the liquid column raised in the tube is equal to the volume of liquid filled
in the cylinder of height h + volume of the liquid filled in the curved surface AEB.
= π r 2 h + (volume of the cylinder ABDC − volume of hemisphere AEB)
2
= π r2 h + � π r2 . r − π r3 �
3
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
Mechanical Properties of Fluids Page 32 of 32
1
= π r2 h + 3 π r3
r
= π r 2 �h + 3�
r
Thus, weight of the liquid column raised in the tube = π r 2 �h + 3� ρg, where ρ is density
of the liquid.
r
In equilibrium, we have 2πr × T cos θ = π r 2 �h + 3� ρg
r
r �h + 3� ρg
T=
2 cos θ
Since h > > r/3, hence r/3 may be neglected in comparison to h. Then
rhρg 2T cos θ
T= ⇒ h=
2 cos θ rρg
RISE OF LIQUID IN AN INCLINED CAPILLARY
The height of liquid column in a glass capillary of radius r is
2T cos θ
h= rρg
where T is surface tension of the liquid and Q is the angle of contact of liquid-glass.
If we incline the capillary tube at an angle α with the vertical, then for balancing the weight
of the liquid column raised in the tube by the force of surface tension, the 'vertical' height of
the liquid in the capillary tube will still be ℎ. For this, the 'length' of the liquid in the tube will
increase to ℎ′, where
ℎ
ℎ′ = cos∝
Substituting the value of h, we have
2T cos θ
h′ =
rρg cos∝
FACTORS AFFECTING SURFACE TENSION
Surface tension is affected by the following factors:
a) Effect of Contamination:
If the water surface has dust, grease or oil, the surface tension of water reduces.
b) Effect of solute:
If the solute is very soluble then the surface tension of liquid increases; as by dissolving
salt in water the surface tension increases. If the solute is less soluble then the surface
tension decreases, as by pouring soap or phenol the surface tension of water decreases.
c) Effect of Temperature :
The surface tension of liquids decreases with rise in temperature.
Offline & Online Tuitions for Grade 9 to 12
Mathematics, Science, Physics, Chemistry
You might also like
- 10-Mechanical Properties of FluidsDocument19 pages10-Mechanical Properties of FluidsHemanthNo ratings yet
- 10-Mechanical Properties of FluidsDocument16 pages10-Mechanical Properties of Fluidsjyothigoudar66No ratings yet
- 10-Mechanical Properties of FluidsDocument20 pages10-Mechanical Properties of FluidsHemanthNo ratings yet
- Fluid MechanicsDocument32 pagesFluid MechanicsShristyNo ratings yet
- Fluid DynamicsDocument24 pagesFluid DynamicsAnupam MNo ratings yet
- Mechanical Properties of FluidsDocument44 pagesMechanical Properties of FluidsG MaheshNo ratings yet
- Fluid MechanicsDocument37 pagesFluid MechanicsabhiNo ratings yet
- Physics Fluid MechanicsDocument18 pagesPhysics Fluid MechanicsPrajwalNo ratings yet
- Fluid Mechanics: Hooke'S LawDocument18 pagesFluid Mechanics: Hooke'S Lawsiddhartha2862No ratings yet
- FLUIDSDocument60 pagesFLUIDSphysics7wallah4No ratings yet
- Fluids: A Fluid Is A Substance That FlowsDocument15 pagesFluids: A Fluid Is A Substance That FlowsValentinaNo ratings yet
- Fluid-Mechanics (1)Document7 pagesFluid-Mechanics (1)Jim Bryan Wabina VergaraNo ratings yet
- Fluid Mechanics Notes & Questions PDFDocument15 pagesFluid Mechanics Notes & Questions PDFJatin SinglaNo ratings yet
- Chapter 15 FluidsDocument21 pagesChapter 15 FluidsVictor ZhaoNo ratings yet
- Fluid Mechanics: PhysicsDocument24 pagesFluid Mechanics: Physicsrudrakshengg.No ratings yet
- Fluid MechanicsDocument16 pagesFluid MechanicsRaju SinghNo ratings yet
- Fluid MechanicsDocument16 pagesFluid MechanicsRaju SinghNo ratings yet
- Fluids Chapter SummaryDocument155 pagesFluids Chapter SummaryFaiyaaz KarimNo ratings yet
- Fluid Mechanics: Key Concepts Exercise-I Exercise-Ii Exercise-Iii Answer KeyDocument16 pagesFluid Mechanics: Key Concepts Exercise-I Exercise-Ii Exercise-Iii Answer Keyrebel rocksNo ratings yet
- Fluids Part1 FinotesDocument21 pagesFluids Part1 Finotespiyushdua01No ratings yet
- Lecture 1-Fluid Statics 2003Document6 pagesLecture 1-Fluid Statics 2003Leonardo LibresNo ratings yet
- Grade 11 Mechanical Properties of FluidsDocument11 pagesGrade 11 Mechanical Properties of FluidsVipin VetriNo ratings yet
- TLS - XI - Physics - Mechanical Properties of FluidsDocument14 pagesTLS - XI - Physics - Mechanical Properties of Fluidsaniruddha deshpandeNo ratings yet
- Chapter Eleven (Fluid Statics)Document16 pagesChapter Eleven (Fluid Statics)ايات امجد امجدNo ratings yet
- Mechanical properties of FluidsDocument16 pagesMechanical properties of FluidsjayaswaleshaanNo ratings yet
- PascalDocument13 pagesPascalPrithviraj RandhawaNo ratings yet
- Xi Physics - Mechanical Properties of FluidsDocument7 pagesXi Physics - Mechanical Properties of FluidsadarshdarasinghNo ratings yet
- 10 - Mechanical Properties of FluidsDocument11 pages10 - Mechanical Properties of Fluidssrujanx360No ratings yet
- Lec. 1Document31 pagesLec. 1Ali. AboudNo ratings yet
- Unit 2-Fluid MechanicsDocument27 pagesUnit 2-Fluid MechanicsApechRanger100% (1)
- Part - 2Document81 pagesPart - 2aryanneet9599No ratings yet
- Chapter 10 Lecture Notes: P GH P GH +P F (A /A B W Q V/ T Av A V A V P V Gy + (1/2) V Gy F Av/lDocument13 pagesChapter 10 Lecture Notes: P GH P GH +P F (A /A B W Q V/ T Av A V A V P V Gy + (1/2) V Gy F Av/lvijayabharathi_vijivijiNo ratings yet
- Modeling Forces Acting On BodyDocument16 pagesModeling Forces Acting On Bodymisael00138868No ratings yet
- 6 Hydro Static DynamicDocument15 pages6 Hydro Static DynamiclundNo ratings yet
- Mechanics 5 FluidDynamicsDocument16 pagesMechanics 5 FluidDynamicsSumiran JaiswalNo ratings yet
- 11 Fluids 1Document13 pages11 Fluids 1Thaya GanapathyNo ratings yet
- Fluid MechanicsDocument34 pagesFluid MechanicsNurulWardhani11No ratings yet
- Fluid ThrustDocument2 pagesFluid ThrustVenu Gopal100% (1)
- Chapter 3 - 2022Document28 pagesChapter 3 - 2022Kibrom FisehaNo ratings yet
- 10 Mechanical Properties of FluidsDocument17 pages10 Mechanical Properties of FluidsPavan KumarNo ratings yet
- Fluid, Osci, Thermo, Waves DeriDocument34 pagesFluid, Osci, Thermo, Waves DeriTech BE SafeNo ratings yet
- Mechanical Properties of Fluids All DervationsDocument12 pagesMechanical Properties of Fluids All DervationsAmiya Kumar Das100% (1)
- Fluid MechanicsDocument58 pagesFluid Mechanicsdinda ayuNo ratings yet
- Fluids Handout Bernoilli and Equation of ContinuityDocument11 pagesFluids Handout Bernoilli and Equation of ContinuityShar anilNo ratings yet
- Fluid Mechanics (MR 231) Lecture Notes (4) : Coefficient of Volume ExpansionDocument11 pagesFluid Mechanics (MR 231) Lecture Notes (4) : Coefficient of Volume ExpansionAhmedTahaNo ratings yet
- Hydrostatic ForcesDocument27 pagesHydrostatic ForcesSumit SahrawatNo ratings yet
- Ch. 4 Pressure in Fluids & Atm Pr.Document13 pagesCh. 4 Pressure in Fluids & Atm Pr.rudranilm2008No ratings yet
- Newton's viscosity law and derivation of expressions for capillary rise and fallDocument10 pagesNewton's viscosity law and derivation of expressions for capillary rise and fallMonith ElyonNo ratings yet
- PressureDocument6 pagesPressuredemetri lanezNo ratings yet
- Class Test 4: 12 May Sem. Tests 1-2 Review: 16 May Semester Test 3: 19 May Tutorial Homework 6: Due 19 MayDocument106 pagesClass Test 4: 12 May Sem. Tests 1-2 Review: 16 May Semester Test 3: 19 May Tutorial Homework 6: Due 19 MayNceba Perseverance MbeweNo ratings yet
- Chapter 10 - Mechanical Properties of FluidsDocument7 pagesChapter 10 - Mechanical Properties of FluidsSk SahilNo ratings yet
- Fluid Mechanics UNIT-1 (Part-2)Document28 pagesFluid Mechanics UNIT-1 (Part-2)Achyutha AnilNo ratings yet
- Hyrdro1 OverheadsDocument43 pagesHyrdro1 OverheadsMukhtarNo ratings yet
- Lecture of Fluid MechanicsDocument42 pagesLecture of Fluid MechanicsAbdullah IrshadNo ratings yet
- Fundamental Fluid Properties ExplainedDocument28 pagesFundamental Fluid Properties ExplainedjohnNo ratings yet
- En-EEM-O1.1 - Expert Time1 - Static FluidsDocument31 pagesEn-EEM-O1.1 - Expert Time1 - Static FluidsMaria-Alejandra GUERRERONo ratings yet
- Fluid StaticsDocument9 pagesFluid StaticsMohammad Zunaied Bin Harun, Lecturer , CEENo ratings yet
- CE 521 - Reaction Paper and Research On Accelerated Liquids in Relatives EquilibriumDocument8 pagesCE 521 - Reaction Paper and Research On Accelerated Liquids in Relatives EquilibriumHipolito RarangolNo ratings yet
- Design of BandharaDocument21 pagesDesign of BandharaNeil Agshikar100% (1)
- PT6A-Series Rigging & Trouble ShootingDocument29 pagesPT6A-Series Rigging & Trouble ShootingDade Sobarna100% (12)
- Hydrostatic Force On Curved SurfacesDocument14 pagesHydrostatic Force On Curved Surfaceseric labordoNo ratings yet
- Design and Fabrication FRP PoolDocument15 pagesDesign and Fabrication FRP Poolm4004No ratings yet
- Pressure Drop Chart Y StrainersDocument1 pagePressure Drop Chart Y StrainersAshimNo ratings yet
- Banco de TubosDocument9 pagesBanco de TubosArturo Arévalo FloresNo ratings yet
- Chapter 9 Flashcards - QuizletDocument3 pagesChapter 9 Flashcards - QuizletYukiko HachiNo ratings yet
- Technical Offer April 2018: Mon Jul 01 12:08:13 2019 Case: T 55.hsc Flowsheet: Case (Main)Document1 pageTechnical Offer April 2018: Mon Jul 01 12:08:13 2019 Case: T 55.hsc Flowsheet: Case (Main)Wael BadriNo ratings yet
- Secondary National Curriculum - Science 220714Document13 pagesSecondary National Curriculum - Science 220714api-237136369No ratings yet
- Manual Instruction CPAM-EKA AIR C16 EKA KOOL V2Document8 pagesManual Instruction CPAM-EKA AIR C16 EKA KOOL V2Tam DuongNo ratings yet
- MCQ BankDocument28 pagesMCQ Banksahulhs100% (6)
- [2nd Month] STM 128 - General Chemistry 2Document36 pages[2nd Month] STM 128 - General Chemistry 2ibnolyn2003No ratings yet
- O Level Physics Pressure NotesDocument28 pagesO Level Physics Pressure NotesMarvel ComicsNo ratings yet
- FR 002 Pressure Testing Inspection ChecklistDocument6 pagesFR 002 Pressure Testing Inspection Checklistum er100% (1)
- BS EN ISO 5175-1 (2017) FBA'sDocument22 pagesBS EN ISO 5175-1 (2017) FBA'sPC100% (1)
- PN1949 Perkins Irrigation Engine Ratings GuideDocument16 pagesPN1949 Perkins Irrigation Engine Ratings GuideMd ShNo ratings yet
- Dyna Drill Spec SheetsDocument105 pagesDyna Drill Spec SheetsDon BraithwaiteNo ratings yet
- Fisher D4 Control Valve ManualDocument24 pagesFisher D4 Control Valve ManualAmiroucheBenlakehalNo ratings yet
- Amca 803Document98 pagesAmca 803insult2injury100% (1)
- Process Heat Transfer Hof MasterDocument327 pagesProcess Heat Transfer Hof MastersdrtfgNo ratings yet
- ME Laboratory 1 - Experiment No. 01Document4 pagesME Laboratory 1 - Experiment No. 01Gabriel Angelo AbrauNo ratings yet
- Design and fabrication of a shock wave generatorDocument6 pagesDesign and fabrication of a shock wave generatoramta nadeemNo ratings yet
- Ce8302 Fluid Mechanics Cat-1 Set-1 FinalDocument3 pagesCe8302 Fluid Mechanics Cat-1 Set-1 Finalyaro oruvanNo ratings yet
- A Hydrostatic Model of The Wirtz Pump - AAMDocument18 pagesA Hydrostatic Model of The Wirtz Pump - AAMKazehaNo ratings yet
- Hydraulic PressesDocument13 pagesHydraulic PressesAhmed Adel ShatlaNo ratings yet
- 5.15 FluidaDocument7 pages5.15 FluidaMuhammadNo ratings yet
- Chapter 2 (Water Pressure)Document12 pagesChapter 2 (Water Pressure)Ship Wonders100% (6)
- RandomDocument1,919 pagesRandomNajmul Puda Pappadam100% (1)
- Aquatherm Pressure Test ProcedureDocument6 pagesAquatherm Pressure Test ProcedureMuhammad AhmedNo ratings yet
- HR Wallingford PDFDocument4 pagesHR Wallingford PDFAzisNo ratings yet
































































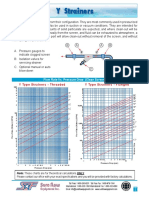






![[2nd Month] STM 128 - General Chemistry 2](https://imgv2-1-f.scribdassets.com/img/document/720602932/149x198/8d406a4ab7/1712413208?v=1)

















