Professional Documents
Culture Documents
KP - FactRecall
KP - FactRecall
Uploaded by
sabinaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
KP - FactRecall
KP - FactRecall
Uploaded by
sabinaCopyright:
Available Formats
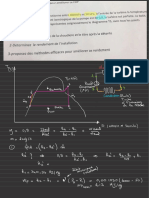
⚖
Equilibrium Constant Kp for Homogenous Systems
> The
equilibrium constant Kp is deduced from the
equation for a reversible reaction
occurring in the
gas phase .
The Effect of Catalyst on Kp
¥7 affect of of not affect
whilst a
catalyst can the rate attainment an
equilibrium ,
it does
Kp .
Definition of Partial Pressure
mole fraction ✗ total pressure
p =
p
=
humberotmoleofgas.AT
-191 number of moles
✗ total pressure
calculating Kp
Big, € yccg, Dig
-
+
WA -1 ✗ -
,
,g ,
↳ =i;¥%:
! Like Kc has
,
only temperature an effect on Kp .
You might also like
- Systematic Approaches To A Successful Literature Review PDFDocument288 pagesSystematic Approaches To A Successful Literature Review PDFعباس معنى100% (1)
- 2014 12 01 Nova Scotia Diligence Report - Web ReadyDocument50 pages2014 12 01 Nova Scotia Diligence Report - Web ReadyheatherloneyNo ratings yet
- Case 31 Digest GR 224498Document1 pageCase 31 Digest GR 224498Lorden Farrel100% (1)
- Panetta: DesignedDocument16 pagesPanetta: DesignedRISHI KACHHADIANo ratings yet
- Chemical Kinetics Class - 7 (Notes)Document26 pagesChemical Kinetics Class - 7 (Notes)ᴜsʜɴᴇᴇᴋNo ratings yet
- EquationsDocument4 pagesEquationsRahul RoopchandNo ratings yet
- 物理冶金2Document1 page物理冶金2游承翰No ratings yet
- Thermodynamics Revision Class-1 Teacher NotesDocument10 pagesThermodynamics Revision Class-1 Teacher Notesvaishyaliya27No ratings yet
- CDocument4 pagesCtheastralxNo ratings yet
- Screenshot 2565-02-15 at 17.46.53Document2 pagesScreenshot 2565-02-15 at 17.46.53Natthanicha THANAPAISANCHOKNo ratings yet
- Chapter 8 1-4Document4 pagesChapter 8 1-4HamiltonNo ratings yet
- HarshDocument20 pagesHarshHarsh Vashishtha67% (3)
- Chemical EquilibriumDocument11 pagesChemical Equilibriumsaurabh dahagaurNo ratings yet
- ThermodynamicsDocument37 pagesThermodynamicsAyushmaan DhanaiNo ratings yet
- EPR 364: Electrical & Electronic Measurements: Lecture 8B Dr. Hussein KotbDocument8 pagesEPR 364: Electrical & Electronic Measurements: Lecture 8B Dr. Hussein Kotbahmed gamalNo ratings yet
- Chemical Equilbrium 2Document4 pagesChemical Equilbrium 2bisenpallavi80No ratings yet
- Transition State With Catalyst G Activation Free Energy G Driving Force GDocument10 pagesTransition State With Catalyst G Activation Free Energy G Driving Force GMir RafsanNo ratings yet
- .9aueñqnbmwñ: Lwsuñn Wñnmi:Ñiuññmq ?FGPÑDocument1 page.9aueñqnbmwñ: Lwsuñn Wñnmi:Ñiuññmq ?FGPÑThaksaporn LeeNo ratings yet
- Physical Race Sol. 1 To 40Document259 pagesPhysical Race Sol. 1 To 40Piyus TopperNo ratings yet
- SolvedProblem PFR VariableVolumeReactionDocument1 pageSolvedProblem PFR VariableVolumeReactionHarish PrasathNo ratings yet
- Larsen & Toubro Limited Kpo Coke Oven Phase-1 LT Cable Sizing Calculation-Bpp AreaDocument12 pagesLarsen & Toubro Limited Kpo Coke Oven Phase-1 LT Cable Sizing Calculation-Bpp AreamustangNo ratings yet
- Behaviour of Gases Mind MapDocument1 pageBehaviour of Gases Mind MapApurv MalviyaNo ratings yet
- Class 12 Physics Derivations Shobhit NirwanDocument24 pagesClass 12 Physics Derivations Shobhit Nirwansuyash KumarNo ratings yet
- Improving Control Valve Performance Chemical Engineering en 127192Document5 pagesImproving Control Valve Performance Chemical Engineering en 127192Adeel Qaiser100% (1)
- Química - LuanaDocument22 pagesQuímica - LuanaCarolNo ratings yet
- Linear Programming: Gas Lift RateDocument7 pagesLinear Programming: Gas Lift RateQuy Tran XuanNo ratings yet
- Pof Only FranceDocument10 pagesPof Only FranceIgnacioNo ratings yet
- FluidsDocument3 pagesFluidsAkshith IsolaNo ratings yet
- General General: Troubleshooting TroubleshootingDocument53 pagesGeneral General: Troubleshooting TroubleshootingHiếu HuỳnhNo ratings yet
- Non-Isothermal Reactor DesignDocument5 pagesNon-Isothermal Reactor Designnorpius7754No ratings yet
- Gaddam, Ikshwak: ResultDocument1 pageGaddam, Ikshwak: Resultpraveen kumarNo ratings yet
- The Effect of Water Influx On P Over Z CurvesDocument5 pagesThe Effect of Water Influx On P Over Z Curvesb mNo ratings yet
- H LawDocument7 pagesH LawAnindya AcharyaNo ratings yet
- CEGB Vol 3 TurbineDocument420 pagesCEGB Vol 3 Turbinesurendra7_14100% (8)
- Isomerism Revision A1aQqazlaOf3gnG1Document42 pagesIsomerism Revision A1aQqazlaOf3gnG1hemantprakash626No ratings yet
- 5T - Engine Supercharging Dr. TarekDocument17 pages5T - Engine Supercharging Dr. TarekOLD GAMESNo ratings yet
- Assignment 9 SolutionsDocument8 pagesAssignment 9 SolutionsClerry SamuelNo ratings yet
- A2 EquilibriumDocument10 pagesA2 Equilibriumdzniz.d10No ratings yet
- Coke Selectivity FundamentalsDocument7 pagesCoke Selectivity Fundamentalssaleh4060No ratings yet
- Assignment 4 FM Intan Nur Haslinda 18001912Document11 pagesAssignment 4 FM Intan Nur Haslinda 18001912Intan NurhaslindaNo ratings yet
- Physics Constants and CoefficientsDocument1 pagePhysics Constants and Coefficientslakshya singhalNo ratings yet
- Ideal GasesDocument13 pagesIdeal Gasesichiwaaa sanNo ratings yet
- Exo Thermos 2emsessDocument12 pagesExo Thermos 2emsessإبراهيم الزايدي الكيحلNo ratings yet
- Prelab 1Document6 pagesPrelab 1YU ZHEN WONGNo ratings yet
- Pharmacodynamics - DRC, Agonist - AntagonistDocument6 pagesPharmacodynamics - DRC, Agonist - AntagonistPriyam Kishore DuttaNo ratings yet
- CE0037L Plate 4 Lateral Loads Format (Perform ALL Grids)Document24 pagesCE0037L Plate 4 Lateral Loads Format (Perform ALL Grids)박용미No ratings yet
- Handnotes Lecture43Document10 pagesHandnotes Lecture43Faheem ShanavasNo ratings yet
- Waves 2Document1 pageWaves 2fghhfgfNo ratings yet
- Approximating Well To Fault Distance From Pressure Build-Up TestsDocument7 pagesApproximating Well To Fault Distance From Pressure Build-Up TestsBolsec14No ratings yet
- CRE Exp3Document4 pagesCRE Exp3kabali007123No ratings yet
- Electrostatics + Magnetostatic + CurrentDocument1 pageElectrostatics + Magnetostatic + Currentrudraveer2805No ratings yet
- COMSOL Bangalore 2019 KishoreSalunkeDocument1 pageCOMSOL Bangalore 2019 KishoreSalunkeLion LionNo ratings yet
- Gas SeparationDocument5 pagesGas SeparationShivanshu BaranwalNo ratings yet
- Correlation of Consolidation Parameters Analysis Based On Cpt-U ResultDocument1 pageCorrelation of Consolidation Parameters Analysis Based On Cpt-U ResultSuHendraNo ratings yet
- Equations of State and PVT AnalysisDocument37 pagesEquations of State and PVT AnalysisJanickNo ratings yet
- Introducción A Los Bioreactores - Reactor BatchDocument14 pagesIntroducción A Los Bioreactores - Reactor BatchMilton SteevenNo ratings yet
- Statics and Strength of M: Aterials Formula SheetDocument1 pageStatics and Strength of M: Aterials Formula Sheetpaolo martin reyesNo ratings yet
- Chapter 5Document28 pagesChapter 5Anthony Leire MontealtoNo ratings yet
- Gas ReservoirDocument2 pagesGas ReservoirDarya Khan BhuttoNo ratings yet
- Eukaryotic Cells - FactRecallDocument3 pagesEukaryotic Cells - FactRecallsabinaNo ratings yet
- Prokaryotic Cells & Viruses - FactRecallDocument4 pagesProkaryotic Cells & Viruses - FactRecallsabinaNo ratings yet
- Cell Cycle - FactRecallDocument2 pagesCell Cycle - FactRecallsabinaNo ratings yet
- Types of Natural Selection - FactRecallDocument1 pageTypes of Natural Selection - FactRecallsabinaNo ratings yet
- RadioactivityDocument7 pagesRadioactivitysabinaNo ratings yet
- Genetic Diversity and Natural Selection - FactRecallDocument2 pagesGenetic Diversity and Natural Selection - FactRecallsabinaNo ratings yet
- Summary of Nephron Action - FactRecallDocument1 pageSummary of Nephron Action - FactRecallsabinaNo ratings yet
- Imat Equation + Formula SheetDocument6 pagesImat Equation + Formula SheetsabinaNo ratings yet
- Osmoregulation - FactRecallDocument3 pagesOsmoregulation - FactRecallsabinaNo ratings yet
- English Certification PaperDocument23 pagesEnglish Certification PapersabinaNo ratings yet
- Value Chain Analysis of Goat in South Omo Zone, SNNPR, EthiopiaDocument13 pagesValue Chain Analysis of Goat in South Omo Zone, SNNPR, EthiopiaPremier PublishersNo ratings yet
- Single Complete DentureDocument36 pagesSingle Complete DentureKhan MksksNo ratings yet
- Material Safety Data Sheet: Revision: Print DateDocument5 pagesMaterial Safety Data Sheet: Revision: Print DatePepabuNo ratings yet
- EDI TBulletin - Factors Influencing Air Requirements To Treat Waste WaterDocument4 pagesEDI TBulletin - Factors Influencing Air Requirements To Treat Waste WaterMonte_CNo ratings yet
- FH Series: 60 HZ 60 HZ 60 HZ 60 HZ 60 HZDocument132 pagesFH Series: 60 HZ 60 HZ 60 HZ 60 HZ 60 HZWilly RiosNo ratings yet
- SONEX Do Wing - Tip - Install Fiberglass Wing TipsDocument1 pageSONEX Do Wing - Tip - Install Fiberglass Wing TipsNZHHNo ratings yet
- ESAB Renegade ES 300i Portable MMA Arc Welder User ManualDocument32 pagesESAB Renegade ES 300i Portable MMA Arc Welder User Manualaxisd47No ratings yet
- NestléDocument2 pagesNestléMariana DíazNo ratings yet
- Botany - 1 (E.m) - 1,2,3,4,5,8,11,13Document149 pagesBotany - 1 (E.m) - 1,2,3,4,5,8,11,13Suresh BabuNo ratings yet
- FMT - Chap 9 - SolutionsDocument6 pagesFMT - Chap 9 - SolutionsVũ Hương ChiNo ratings yet
- Msds o Xylene PDFDocument6 pagesMsds o Xylene PDFPriska Dewi AnjarsariNo ratings yet
- BURNSDocument17 pagesBURNSUsaid SulaimanNo ratings yet
- HYPERLIGHT - A Breakthrough in MedicineDocument12 pagesHYPERLIGHT - A Breakthrough in MedicinePeter CsalloNo ratings yet
- IandF SA3 201509 ExaminersReportDocument23 pagesIandF SA3 201509 ExaminersReportdickson phiriNo ratings yet
- CoA PMA600HD GBDocument19 pagesCoA PMA600HD GBLuiz Marcos De OliveiraNo ratings yet
- Salwico IR Test Lamp: IR Flame Detector TesterDocument1 pageSalwico IR Test Lamp: IR Flame Detector TesterТарас АртюхNo ratings yet
- Application Guide: Clean in Place Conductivity Monitoring SystemsDocument4 pagesApplication Guide: Clean in Place Conductivity Monitoring Systemspatitay036817No ratings yet
- Vs-Vsk.230..Pbf Series: Vishay SemiconductorsDocument9 pagesVs-Vsk.230..Pbf Series: Vishay SemiconductorsrenidwilNo ratings yet
- Technical Data Sheet: H.E.N.E. Hard Chrome ProcessDocument5 pagesTechnical Data Sheet: H.E.N.E. Hard Chrome ProcessLuuThiThuyDuong100% (1)
- Contact NumbersDocument1 pageContact Numbers3 SR Welfare TeamNo ratings yet
- ISO 4020 ExtractDocument2 pagesISO 4020 ExtractBartek HajaNo ratings yet
- 2019 Sec 4 English SA1 St. Margaret's Secondary AnswerDocument10 pages2019 Sec 4 English SA1 St. Margaret's Secondary AnswerKairo ItsukiNo ratings yet
- 10 Contoh Teks Short 'Story Telling' Pendek Bahasa Inggris Unik Dan MenarikDocument13 pages10 Contoh Teks Short 'Story Telling' Pendek Bahasa Inggris Unik Dan MenarikDard TongNo ratings yet
- Medication Information For Parents and Teachers: Carbamazepine-Tegretol, Carbatrol, Epitol, Equetro, Tegretol XRDocument8 pagesMedication Information For Parents and Teachers: Carbamazepine-Tegretol, Carbatrol, Epitol, Equetro, Tegretol XRMonique WrightNo ratings yet
- Brosur Zurich Travel Insurance English VersionDocument2 pagesBrosur Zurich Travel Insurance English VersionDhiany Nadya UtamiNo ratings yet
- Hospital Document List of Procedures For ANHDocument16 pagesHospital Document List of Procedures For ANHsrisaravanan67% (3)
- Calorific Value and Bomb's CalorimeterDocument10 pagesCalorific Value and Bomb's Calorimeterparvezalamkhan62% (13)